Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning
- PMID: 19001324
- PMCID: PMC2645102
- DOI: 10.1200/JCO.2008.17.7279
Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning
Abstract
Purpose: Inhibitors of the mammalian target of rapamycin (mTOR) kinase have shown clinical activity in several lymphoma subtypes. Sirolimus, an mTOR inhibitor, also has activity in the treatment and prophylaxis of graft-versus-host disease (GVHD) after allogeneic hematopoietic stem-cell transplantation (HSCT). We hypothesized that the use of sirolimus for GVHD prophylaxis in patients with lymphoma might lead to improved survival after transplantation through a decreased incidence of disease progression.
Patients and methods: We retrospectively analyzed 190 patients who underwent transplantation for lymphoma. We compared the outcomes of patients who received sirolimus for GVHD prophylaxis with those of patients who received transplantation with a combination of a calcineurin inhibitor and methotrexate without sirolimus.
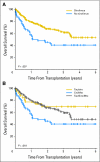
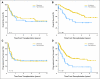
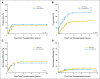
Results: Overall survival (OS) after transplantation was significantly superior in the sirolimus group, which was confirmed in multivariable analysis. The benefit was restricted to patients undergoing reduced-intensity conditioning (RIC) HSCT (3-year OS, 66% for sirolimus group v 38% for no-sirolimus group; P = .007; hazard ratio [HR] for mortality in multivariable analysis = 0.5, P = .042). Patients who received sirolimus had a similar incidence of nonrelapse mortality but a decreased incidence of disease progression compared with patients who did not receive sirolimus (3-year cumulative incidence of progression, 42% v 74%, respectively; P < .001; HR for progression in multivariable analysis = 0.4, P = .01). The effect of sirolimus persisted after adjusting for the occurrence of GVHD. No such survival advantage was apparent in a similar comparison of patients who underwent transplantation for diseases other than lymphoma.
Conclusion: This study suggests that sirolimus can independently decrease the risk of lymphoma progression after RIC HSCT, paving the way for prospective clinical trials.
Figures
Comment in
-
Sirolimus-containing graft-versus-host disease prophylaxis in patients with lymphoma.J Clin Oncol. 2009 Oct 1;27(28):e138; author reply e139-40. doi: 10.1200/JCO.2009.23.9483. Epub 2009 Aug 31. J Clin Oncol. 2009. PMID: 19720890 No abstract available.
References
-
- Sehgal SN: Sirolimus: Its discovery, biological properties, and mechanism of action. Transplant Proc 35:7S–14S, 2003. (suppl) - PubMed
-
- Sehgal SN: Rapamune (RAPA, rapamycin, sirolimus): Mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem 31:335-340, 1998 - PubMed
-
- Mehrabi A, Fonouni H, Kashfi A, et al: The role and value of sirolimus administration in kidney and liver transplantation. Clin Transplant 20:30-43, 2006. (suppl 17) - PubMed
-
- Benito AI, Furlong T, Martin PJ, et al: Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease. Transplantation 72:1924-1929, 2001 - PubMed
-
- Johnston LJ, Brown J, Shizuru JA, et al: Rapamycin (sirolimus) for treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant 11:47-55, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

