Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741
- PMID: 19001325
- PMCID: PMC2645101
- DOI: 10.1200/JCO.2008.17.7147
Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741
Abstract
Purpose: In this report, we update survival (OS) and time-to-progression (TTP) data for the Intergroup trial N9741 after a median 5 years of follow-up by using risk-stratified and prognostic factor analyses to determine if treatment outcomes differ in specific patient subgroups.
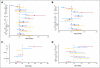
Patients and methods: A total of 1,691 patients were randomly assigned to one of seven fluorouracil-, oxaliplatin-, and irinotecan-containing regimens. OS and TTP were calculated by treatment arm and baseline risk group (on the basis of WBC, performance status, number of sites of disease, and alkaline phosphatase). Multivariate prognostic factor analysis was used to assess clinical factors for their relationships to OS, TTP, response, and toxicity by using Cox and logistic regression models.
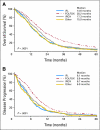
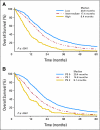
Results: The observed 5-year survival with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX) of 9.8% was better than with irinotecan plus bolus fluorouracil and leucovorin (IFL; 3.7%; P = .04) or with bolus irinotecan/oxaliplatin (IROX; 5.1%; P = .128). OS and TTP were significantly longer for FOLFOX (20.2 months and 8.9 months, respectively) than for IFL (14.6 months and 6.1 months, respectively; P < .001 for both) or for IROX (17.3 months and 6.7 months, respectively; P < .001 for both). OS differed by risk group: 20.7 months for low risk, 17.4 months for intermediate risk, and 9.4 months for high risk (P < .001). FOLFOX treatment was superior in all risk groups and was the most powerful prognostic factor for OS, TTP, response rate, and toxicity.
Conclusion: The 9.8% 5-year OS in patients with metastatic colorectal cancer who were treated with first-line FOLFOX sets a new benchmark. Neither baseline risk group nor any prognostic factor examined was predictive of treatment-specific outcome. However, treatment efficacy and patient longevity varied as a function of risk group.
Figures
References
-
- Goldberg RM, Sargent DJ, Morton RF, et al: A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23-30, 2004 - PubMed
-
- Köhne CH, Cunningham D, Di CF, et al: Clinical determinants of survival in patients with 5-fluorouracil–based treatment for metastatic colorectal cancer: Results of a multivariate analysis of 3825 patients. Ann Oncol 13:308-317, 2002 - PubMed
-
- Kent DM, Hayward RA: Limitations of applying summary results of clinical trials to individual patients: The need for risk stratification. JAMA 298:1209-1212, 2007 - PubMed
-
- Delaunoit T, Goldberg RM, Sargent DJ, et al: Mortality associated with daily bolus 5-fluorouracil/leucovorin administered in combination with either irinotecan or oxaliplatin: Results from Intergroup Trial N9741. Cancer 101:2170-2176, 2004 - PubMed
-
- Benson AB 3rd, Read TR, Goebel SL, Koeller JM, Tormey DC: Correlations between leukocyte count and absolute granulocyte count in patients receiving cancer chemotherapy. Cancer 56:1350-1355, 1985 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

