Denervation induces cytosolic phospholipase A2-mediated fatty acid hydroperoxide generation by muscle mitochondria
- PMID: 19001413
- PMCID: PMC2610521
- DOI: 10.1074/jbc.M806311200
Denervation induces cytosolic phospholipase A2-mediated fatty acid hydroperoxide generation by muscle mitochondria
Abstract
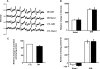
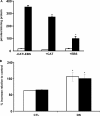
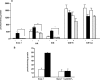
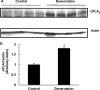
Previously, we demonstrated that mitochondria from denervated muscle exhibited dramatically higher Amplex Red dependent fluorescence (thought to be highly specific for hydrogen peroxide) compared with control muscle mitochondria. We now demonstrate that catalase only partially inhibits the Amplex Red signal in mitochondria from denervated muscle. In contrast, ebselen (a glutathione peroxidase mimetic and inhibitor of fatty acid hydroperoxides) significantly inhibits the Amplex Red signal. This suggests that the majority of the Amplex Red signal in mitochondria from denervated muscle is not derived from hydrogen peroxide. Because Amplex Red cannot react with substrates in the lipid environment, we hypothesize that lipid hydroperoxides formed within the mitochondrial lipid bilayer are released as fatty acid hydroperoxides and react with the Amplex Red probe. We also suggest that the release of fatty acid hydroperoxides from denervated muscle mitochondria may be an important determinant of muscle atrophy. In support of this, muscle atrophy and the Amplex Red signal are inhibited in caloric restricted mice and in transgenic mice that overexpress the lipid hydroperoxide-detoxifying enzyme glutathione peroxidase 4. Finally, we propose that cytosolic phospholipase A2 may be a potential source of these hydroperoxides.
Figures
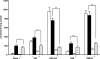
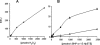
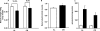
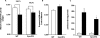

References
-
- Karakelides, H., and Nair, K. S. (2005) Curr. Top Dev. Biol. 68 123–148 - PubMed
-
- Greenlund, L. J., and Nair, K. S. (2003) Mech. Ageing Dev. 124 287–299 - PubMed
-
- Doherty, T. J., Vandervoort, A. A., Taylor, A. W., and Brown, W. F. (1993) J. Appl. Physiol. 74 868–874 - PubMed
-
- Thomason, D. B., and Booth, F. W. (1990) J. Appl. Physiol. 68 1–12 - PubMed
-
- Delbono, O. (2003) Aging Cell 2 21–29 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

