A novel role of Shc adaptor proteins in steroid hormone-regulated cancers
- PMID: 19001530
- PMCID: PMC2776657
- DOI: 10.1677/ERC-08-0179
A novel role of Shc adaptor proteins in steroid hormone-regulated cancers
Abstract
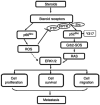
Tyrosine phosphorylation plays a critical role in growth regulation, and its aberrant regulation can be involved in carcinogenesis. The association of Shc (Src homolog and collagen homolog) adaptor protein family members in tyrosine phosphorylation signaling pathway is well recognized. Shc adaptor proteins transmit activated tyrosine phosphorylation signaling that suggest their plausible role in growth regulation including carcinogenesis and metastasis. In parallel, by sharing a similar mechanism of carcinogenesis, the steroids are involved in the early stage of carcinogenesis as well as the regulation of cancer progression and metastatic processes. Recent evidence indicates a cross-talk between tyrosine phosphorylation signaling and steroid hormone action in epithelial cells, including prostate and breast cancer cells. Therefore, the members of Shc proteins may function as mediators between tyrosine phosphorylation and steroid signaling in steroid-regulated cell proliferation and carcinogenesis. In this communication, we discuss the novel roles of Shc proteins, specifically p52(Shc) and p66(Shc), in steroid hormone-regulated cancers and a novel molecular mechanism by which redox signaling induced by p66(Shc) mediates steroid action via a non-genomic pathway. The p66(Shc) protein may serve as an effective biomarker for predicting cancer prognosis as well as a useful target for treatment.
Conflict of interest statement
All authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures
References
-
- Abdollahi A, Gruver BN, Patriotis C, Hamilton TC. Identification of epidermal growth factor-responsive genes in normal rat ovarian surface epithelial cells. Biochemical and Biophysical Research Communications. 2003;18:188–197. - PubMed
-
- Alzamora R, Harvey BJ. Direct binding and activation of protein kinase C isoforms by steroid hormones. Steroids. 2008;73:885–888. - PubMed
-
- Anand-Apte B, Zetter BR, Viswanathan A, Qiu RG, Chen J, Ruggieri R, Symons M. Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. Journal of Biological Chemistry. 1997;272:30688–30692. - PubMed
-
- Armen TA, Gay CV. Simultaneous detection and functional response of testosterone and estradiol receptors in osteoblast plasma membranes. Journal of Cellular Biochemistry. 2000;79:620–627. - PubMed
-
- Benten WP, Lieberherr M, Giese G, Wunderlich F. Estradiol binding to cell surface raises cytosolic free calcium in T cells. FEBS Letters. 1998;422:349–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

