Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells
- PMID: 19001604
- PMCID: PMC2720766
- DOI: 10.1093/jnci/djn378
Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells
Abstract
Background: Lysophosphatidic acid (LPA) acts through the cell surface G protein-coupled receptors, LPA1, LPA2, or LPA3, to elicit a wide range of cellular responses. It is present at high levels in intraperitoneal effusions of human ovarian cancer increasing cell survival, proliferation, and motility as well as stimulating production of neovascularizing factors. LPA2 and LPA3 and enzymes regulating the production and degradation of LPA are aberrantly expressed by ovarian cancer cells, but the consequences of these expression changes in ovarian cancer cells were unknown.
Methods: Expression of LPA1, LPA2, or LPA3 was inhibited or increased in ovarian cancer cells using small interfering RNAs (siRNAs) and lentivirus constructs, respectively. We measured the effects of changes in LPA receptor expression on cell proliferation (by crystal violet staining), cell motility and invasion (using Boyden chambers), and cytokines (interleukin 6 [IL-6], interleukin 8 [IL-8], and vascular endothelial growth factor [VEGF]) production by enzyme-linked immunosorbent assay. The role of LPA receptors in tumor growth, ascites formation, and cytokine production was assessed in a mouse xenograft model. All statistical tests were two-sided.
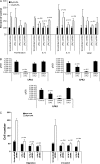
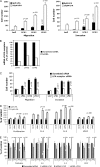
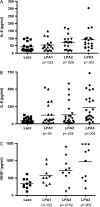
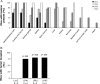
Results: SKOV-3 cells with increased expression of LPA receptors showed increased invasiveness, whereas siRNA knockdown inhibited both migration (P < .001, Student t test) and invasion. Knockdown of the LPA2 or LPA3 receptors inhibited the production of IL-6, IL-8, and VEGF in SKOV-3 and OVCAR-3 cells. SKOV-3 xenografts expressing LPA receptors formed primary tumors of increased size and increased ascites volume. Invasive tumors in the peritoneal cavity occurred in 75% (n = 4) of mice injected with LPA1 expressing SKOV-3 and 80% (n = 5) of mice injected with LPA2 or LPA3 expressing SKOV-3 cells. Metastatic tumors expressing LPA1, LPA2, and LPA3 were identified in the liver, kidney, and pancreas; tumors expressing LPA2 and LPA3 were detected in skeletal muscle; and tumors expressing LPA2 were also found in the cervical lymph node and heart. The percent survival of mice with tumors expressing LPA2 or LPA3 was reduced in comparison with animals with tumors expressing beta-galactosidase.
Conclusions: Expression of LPA2 or LPA3 during ovarian carcinogenesis contributes to ovarian cancer aggressiveness, suggesting that the targeting of LPA production and action may have potential for the treatment of ovarian cancer.
Figures


Similar articles
-
Regulator of G-protein signalling expression and function in ovarian cancer cell lines.Cell Mol Biol Lett. 2009;14(1):153-74. doi: 10.2478/s11658-008-0040-7. Epub 2008 Oct 31. Cell Mol Biol Lett. 2009. PMID: 18979070 Free PMC article.
-
LPA2 (EDG4) mediates Rho-dependent chemotaxis with lower efficacy than LPA1 (EDG2) in breast carcinoma cells.Am J Physiol Cell Physiol. 2007 May;292(5):C1927-33. doi: 10.1152/ajpcell.00400.2006. Am J Physiol Cell Physiol. 2007. PMID: 17496233
-
Differential targeting of lysophosphatidic acid LPA1, LPA2, and LPA3 receptor signalling by tricyclic and tetracyclic antidepressants.Eur J Pharmacol. 2023 Nov 15;959:176064. doi: 10.1016/j.ejphar.2023.176064. Epub 2023 Sep 25. Eur J Pharmacol. 2023. PMID: 37758013
-
Two pathways for lysophosphatidic acid production.Biochim Biophys Acta. 2008 Sep;1781(9):513-8. doi: 10.1016/j.bbalip.2008.06.005. Epub 2008 Jun 24. Biochim Biophys Acta. 2008. PMID: 18621144 Review.
-
Significance of vascular endothelial growth factor in growth and peritoneal dissemination of ovarian cancer.Cancer Metastasis Rev. 2012 Jun;31(1-2):143-62. doi: 10.1007/s10555-011-9337-5. Cancer Metastasis Rev. 2012. PMID: 22101807 Free PMC article. Review.
Cited by
-
Friend and foe: the regulation network of ascites components in ovarian cancer progression.J Cell Commun Signal. 2023 Sep;17(3):391-407. doi: 10.1007/s12079-022-00698-8. Epub 2022 Oct 13. J Cell Commun Signal. 2023. PMID: 36227507 Free PMC article. Review.
-
Regulation of tumor cell - Microenvironment interaction by the autotaxin-lysophosphatidic acid receptor axis.Adv Biol Regul. 2019 Jan;71:183-193. doi: 10.1016/j.jbior.2018.09.008. Epub 2018 Sep 16. Adv Biol Regul. 2019. PMID: 30243984 Free PMC article. Review.
-
Hax-1 is required for Rac1-Cortactin interaction and ovarian carcinoma cell migration.Genes Cancer. 2014 Mar;5(3-4):84-99. doi: 10.18632/genesandcancer.8. Genes Cancer. 2014. PMID: 25053987 Free PMC article.
-
Application of Nanoparticles for Targeting G Protein-Coupled Receptors.Int J Mol Sci. 2018 Jul 10;19(7):2006. doi: 10.3390/ijms19072006. Int J Mol Sci. 2018. PMID: 29996469 Free PMC article. Review.
-
Lipase mimetic cyclodextrins.Chem Sci. 2020 Nov 19;12(3):1090-1094. doi: 10.1039/d0sc05711h. Chem Sci. 2020. PMID: 34163875 Free PMC article.
References
-
- Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev. 2003;3(8):582–591. - PubMed
-
- Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26(8):870–881. - PubMed
-
- An S, Bleu T, Hallmark OG, Goetzl EJ. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J Biol Chem. 1998;273(14):7906–7910. - PubMed
-
- Bandoh K, Aoki J, Hosono H, et al. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J Biol Chem. 1999;274(39):27776–27785. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

