NFBD1/MDC1, 53BP1 and BRCA1 have both redundant and unique roles in the ATM pathway
- PMID: 19001859
- PMCID: PMC2763172
- DOI: 10.4161/cc.7.22.7102
NFBD1/MDC1, 53BP1 and BRCA1 have both redundant and unique roles in the ATM pathway
Abstract
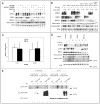
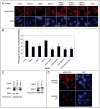
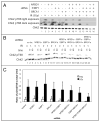
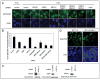
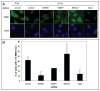
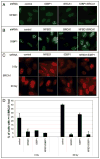
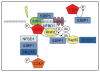
NFBD1/MDC1, 53BP1 and BRCA1 are DNA damage checkpoint proteins with twin BRCT domains. In order to determine if they have redundant roles in responses to ionizing radiation, we used siRNA and shRNA to deplete NFBD1, 53BP1 and BRCA1 in single, double and triple combinations. These analyses were performed in early passage human foreskin fibroblasts so that checkpoint responses could be assessed in a normal genetic background. We report that NFBD1, 53BP1 and BRCA1 have both unique and redundant functions in radiation-induced phosphorylation and localization events in the ATM-Chk2 pathway. 53BP1, but not NFBD1 and BRCA1, mediates ionizing radiation-induced ATM S1981 autophosphorylation. In contrast, all three mediators collaborate to promote IR-induced Chk2 T68 phosphorylation. NFBD1 and 53BP1, but not BRCA1, work together to mediate pATMS1981, pChk2T68 and NBS1 ionizing radiation induced foci (IRIF). However, the relative importance of NFBD1 and 53BP1 in IRIF formation differ. We also determined the interdependence among mediators in IRIF recruitment. We extend previous findings in cancer cells and mouse cells that NFBD1 is upstream of 53BP1 and BRCA1 to primary human cells. Furthermore, NFBD1 promotes BRCA1 IRIF through both 53BP1-dependent and 53BP1-independent mechanisms.
Figures
Similar articles
-
53BP1 and NFBD1/MDC1-Nbs1 function in parallel interacting pathways activating ataxia-telangiectasia mutated (ATM) in response to DNA damage.Cancer Res. 2003 Dec 15;63(24):8586-91. Cancer Res. 2003. PMID: 14695167
-
NFBD1/Mdc1 mediates ATR-dependent DNA damage response.Cancer Res. 2005 Feb 15;65(4):1158-63. doi: 10.1158/0008-5472.CAN-04-2508. Cancer Res. 2005. PMID: 15734998
-
NFBD1, like 53BP1, is an early and redundant transducer mediating Chk2 phosphorylation in response to DNA damage.J Biol Chem. 2003 Mar 14;278(11):8873-6. doi: 10.1074/jbc.C300001200. Epub 2003 Jan 24. J Biol Chem. 2003. PMID: 12551934
-
MDC1/NFBD1: a key regulator of the DNA damage response in higher eukaryotes.DNA Repair (Amst). 2004 Aug-Sep;3(8-9):953-7. doi: 10.1016/j.dnarep.2004.03.007. DNA Repair (Amst). 2004. PMID: 15279781 Review.
-
The influence of heterochromatin on DNA double strand break repair: Getting the strong, silent type to relax.DNA Repair (Amst). 2010 Dec 10;9(12):1273-82. doi: 10.1016/j.dnarep.2010.09.013. Epub 2010 Oct 30. DNA Repair (Amst). 2010. PMID: 21036673 Review.
Cited by
-
Epigenetic regulation of genomic integrity.Chromosoma. 2012 Apr;121(2):131-51. doi: 10.1007/s00412-011-0358-1. Epub 2012 Jan 17. Chromosoma. 2012. PMID: 22249206 Free PMC article. Review.
-
NFBD1/MDC1 regulates Cav1 and Cav2 independently of DNA damage and p53.Mol Cancer Res. 2011 Jun;9(6):766-81. doi: 10.1158/1541-7786.MCR-10-0317. Epub 2011 May 6. Mol Cancer Res. 2011. PMID: 21551225 Free PMC article.
-
The Role of Nitric Oxide in Cancer: Master Regulator or NOt?Int J Mol Sci. 2020 Dec 10;21(24):9393. doi: 10.3390/ijms21249393. Int J Mol Sci. 2020. PMID: 33321789 Free PMC article. Review.
-
A novel MCPH1 isoform complements the defective chromosome condensation of human MCPH1-deficient cells.PLoS One. 2012;7(8):e40387. doi: 10.1371/journal.pone.0040387. Epub 2012 Aug 30. PLoS One. 2012. PMID: 22952573 Free PMC article.
-
Sinomenine hydrochloride sensitizes cervical cancer cells to ionizing radiation by impairing DNA damage response.Oncol Rep. 2018 Nov;40(5):2886-2895. doi: 10.3892/or.2018.6693. Epub 2018 Sep 10. Oncol Rep. 2018. PMID: 30226618 Free PMC article.
References
-
- Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. - PubMed
-
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. - PubMed
-
- Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 2000;60:5934–6. - PubMed
-
- Melchionna R, Chen XB, Blasina A, McGowan CH. Threonine 68 is required for radiation-induced phosphorylation and activation of Cds1. Nat Cell Biol. 2000;2:762–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
