Gene-mediated restoration of normal myofiber elasticity in dystrophic muscles
- PMID: 19002166
- PMCID: PMC2834969
- DOI: 10.1038/mt.2008.239
Gene-mediated restoration of normal myofiber elasticity in dystrophic muscles
Abstract
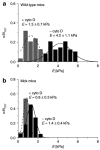
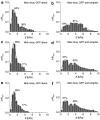
Dystrophin mediates a physical link between the cytoskeleton of muscle fibers and the extracellular matrix, and its absence leads to muscle degeneration and dystrophy. In this article, we show that the lack of dystrophin affects the elasticity of individual fibers within muscle tissue explants, as probed using atomic force microscopy (AFM), providing a sensitive and quantitative description of the properties of normal and dystrophic myofibers. The rescue of dystrophin expression by exon skipping or by the ectopic expression of the utrophin analogue normalized the elasticity of dystrophic muscles, and these effects were commensurate to the functional recovery of whole muscle strength. However, a more homogeneous and widespread restoration of normal elasticity was obtained by the exon-skipping approach when comparing individual myofibers. AFM may thus provide a quantification of the functional benefit of gene therapies from live tissues coupled to single-cell resolution.
Figures



Similar articles
-
Combined effect of AAV-U7-induced dystrophin exon skipping and soluble activin Type IIB receptor in mdx mice.Hum Gene Ther. 2012 Dec;23(12):1269-79. doi: 10.1089/hum.2012.056. Epub 2012 Sep 24. Hum Gene Ther. 2012. PMID: 22894762
-
Muscle function recovery in golden retriever muscular dystrophy after AAV1-U7 exon skipping.Mol Ther. 2012 Nov;20(11):2120-33. doi: 10.1038/mt.2012.181. Epub 2012 Sep 11. Mol Ther. 2012. PMID: 22968479 Free PMC article.
-
C-terminal-truncated microdystrophin recruits dystrobrevin and syntrophin to the dystrophin-associated glycoprotein complex and reduces muscular dystrophy in symptomatic utrophin/dystrophin double-knockout mice.Mol Ther. 2006 Jul;14(1):79-87. doi: 10.1016/j.ymthe.2006.01.007. Epub 2006 Mar 23. Mol Ther. 2006. PMID: 16563874 Free PMC article.
-
[Gene therapy for muscular dystrophy].No To Hattatsu. 2004 Mar;36(2):117-23. No To Hattatsu. 2004. PMID: 15031985 Review. Japanese.
-
Progress toward Gene Therapy for Duchenne Muscular Dystrophy.Mol Ther. 2017 May 3;25(5):1125-1131. doi: 10.1016/j.ymthe.2017.02.019. Epub 2017 Apr 15. Mol Ther. 2017. PMID: 28416280 Free PMC article. Review.
Cited by
-
Three-dimensional mechanical characterization of murine skeletal muscle using quantitative micro-elastography.Biomed Opt Express. 2022 Oct 17;13(11):5879-5899. doi: 10.1364/BOE.471062. eCollection 2022 Nov 1. Biomed Opt Express. 2022. PMID: 36733728 Free PMC article.
-
Exacerbation of pathology by oxidative stress in respiratory and locomotor muscles with Duchenne muscular dystrophy.J Physiol. 2011 May 1;589(Pt 9):2161-70. doi: 10.1113/jphysiol.2011.207456. Epub 2011 Mar 8. J Physiol. 2011. PMID: 21486793 Free PMC article. Review.
-
Fibrogenesis in LAMA2-Related Muscular Dystrophy Is a Central Tenet of Disease Etiology.Front Mol Neurosci. 2020 Feb 4;13:3. doi: 10.3389/fnmol.2020.00003. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32116541 Free PMC article. Review.
-
An AFM-Based Nanomechanical Study of Ovarian Tissues with Pathological Conditions.Int J Nanomedicine. 2020 Jun 19;15:4333-4350. doi: 10.2147/IJN.S254342. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32606681 Free PMC article.
-
Conformational dynamics of actin: effectors and implications for biological function.Cytoskeleton (Hoboken). 2010 Oct;67(10):609-29. doi: 10.1002/cm.20473. Cytoskeleton (Hoboken). 2010. PMID: 20672362 Free PMC article. Review.
References
-
- Cohn RD., and , Campbell KP. Molecular basis of muscular dystrophies. Muscle Nerve. 2000;23:1456–1467. - PubMed
-
- Ikai A., and , Afrin R. Toward mechanical manipulations of cell membranes and membrane proteins using an atomic force microscope. Cell Biochem Biophys. 2003;39:257–277. - PubMed
-
- Dorchies OM, Wagner S, Vuadens O, Waldhauser K, Buetler TM, Kucera P, et al. Green tea extract and its major polyphenol (-)-epigallocatechin gallate improve muscle function in a mouse model for Duchenne muscular dystrophy. Am J Physiol Cell Physiol. 2006;290:C616–C625. - PubMed
-
- Koenig M, Monaco AP., and , Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:209–227. - PubMed
-
- Blake DJ, Weir A, Newey SE., and , Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

