XeNA: capecitabine plus docetaxel, with or without trastuzumab, as preoperative therapy for early breast cancer
- PMID: 19002271
- PMCID: PMC2581822
- DOI: 10.7150/ijms.5.341
XeNA: capecitabine plus docetaxel, with or without trastuzumab, as preoperative therapy for early breast cancer
Abstract
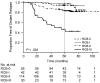
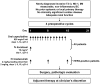
Combinations of capecitabine and a taxane are highly active in metastatic breast cancer, and synergy between capecitabine and docetaxel has also been demonstrated. Such combinations potentially would provide a promising non-anthracycline-based alternative for patients with early breast cancer. Non-anthracycline preoperative regimens are a particularly interesting proposition in human epidermal growth factor receptor 2 (HER2)-positive breast cancer, as they offer less cardiotoxicity and thus can be used concomitantly with preoperative trastuzumab therapy. Capecitabine plus docetaxel (XT) and trastuzumab with XT (HXT) are promising non-anthracycline regimens for the preoperative treatment of women with HER2-negative and HER2-positive breast cancer, respectively. The Xeloda in Neoadjuvant (XeNA) trial, an open-label, multicenter, phase II study, independently assesses the efficacy of preoperative XT in HER2-negative and HXT in HER2-positive breast cancer. A particularly important feature of the XeNA study is the use of pathologic complete response (pCR) plus near pCR (npCR) as the primary endpoint. pCR is associated with long-term survival, and although it is valuable as a surrogate marker, pCR has some limitations. Measurement of residual breast cancer burden (RCB) has been proposed as a more practical alternative to predict survival after preoperative chemotherapy. The combination of RCB-0 and RCB-I (npCR) expands the subset of patients shown to benefit from preoperative chemotherapy, and achievement of pCR or npCR is associated with long disease-free survival. In XeNA, the sum of pCR and npCR will facilitate correlative studies designed to identify patients most likely to benefit from XT and HXT and may expedite the clinical evaluation of these novel preoperative regimens.
Keywords: Anthracycline-induced cardiotoxicity; Breast-conserving surgery; Pathologic complete response; Taxane.
Conflict of interest statement
CONFLICT OF INTEREST: Dr. Gluck received honoraria, consultant and research funding for this study from Roche, Genentech, and Sanofi-Aventis. Edward F. McKenna, Jr, PharmD, is an employee of Roche (Associate Medical Director, Oncology). Dr. Royce received grant support from Roche and Genentech and is a member of the speaker's bureau for Genentech and Roche.
Figures
References
-
- Kaufmann M, Hortobagyi GN, Goldhirsch A et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006;24:1940–1949. - PubMed
-
- Fisher B, Bryant J, Wolmark N et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. - PubMed
-
- Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:188–94. - PubMed
-
- Sachelarie I, Grossbard ML, Chadha M et al. Primary systemic therapy of breast cancer. Oncologist. 2006;11:574–589. - PubMed
-
- Bonadonna G, Valagussa P, Brambilla C et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol. 1998;16:93–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

