Separation of CHO cells using hydrocyclones
- PMID: 19002842
- PMCID: PMC2151964
- DOI: 10.1007/s10616-007-9108-x
Separation of CHO cells using hydrocyclones
Abstract
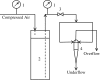
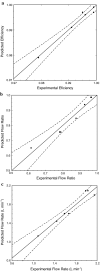
Hydrocyclones are simple and robust separation devices with no moving parts. In the past few years, their use in animal cell separation has been proposed. In this work, the use of different hydrocyclone configurations for Chinese hamster ovary (CHO) cell separation was investigated following an experimental design. It was shown that cell separation efficiencies for cultures of the wild-type CHO.K1 cell line and of a recombinant CHO cell line producing granulocyte-macrophage colony stimulating factor (GM-CSF) were kept above 97%. Low viability losses were observed, as measured by trypan blue exclusion and by determination of intracellular lactate dehydrogenase (LDH) released to the culture medium. Mathematical models were proposed to predict the flow rate, flow ratio and separation efficiency as a function of hydrocyclone geometry and pressure drop. When cells were monitored for any induction of apoptosis upon passage through the hydrocyclones, no increase in apoptotic cell concentration was observed within 48 h of hydrocycloning. Thus, based on the high separation efficiencies, the robustness of the equipment, and the absence of apoptosis induction, hydrocyclones seem to be specially suited for use as cell retention devices in long-term perfusion runs.
Figures



Similar articles
-
Hydrocyclones as cell retention device for CHO perfusion processes in single-use bioreactors.Biotechnol Bioeng. 2020 Jul;117(7):1915-1928. doi: 10.1002/bit.27335. Epub 2020 Apr 8. Biotechnol Bioeng. 2020. PMID: 32181883
-
Application of hydrocyclone for separation of Pichia pastoris produced r-HBsAg from fermentation culture: impact of concentration and pressure on hydrocyclone performance.Prep Biochem Biotechnol. 2019;49(8):813-821. doi: 10.1080/10826068.2019.1621891. Epub 2019 Jun 6. Prep Biochem Biotechnol. 2019. PMID: 31169457
-
Hydrocyclones for the separation of impurities in pretreated biowaste.Waste Manag. 2017 Jun;64:12-19. doi: 10.1016/j.wasman.2017.03.001. Epub 2017 Mar 13. Waste Manag. 2017. PMID: 28302525
-
Application of a 3D printed miniaturized hydrocyclone in biopharmaceutical industry-numerical and experimental studies of yeast separation from fermentation culture media.Prep Biochem Biotechnol. 2023;53(1):31-39. doi: 10.1080/10826068.2022.2035746. Epub 2022 Feb 28. Prep Biochem Biotechnol. 2023. PMID: 35225162
-
Hydrodynamic Evaluation of a Filtering Hydrocyclone for Solid Particle/Water Separation.Membranes (Basel). 2024 Aug 6;14(8):171. doi: 10.3390/membranes14080171. Membranes (Basel). 2024. PMID: 39195423 Free PMC article.
Cited by
-
Integration of Aqueous Two-Phase Extraction as Cell Harvest and Capture Operation in the Manufacturing Process of Monoclonal Antibodies.Antibodies (Basel). 2017 Dec 1;6(4):21. doi: 10.3390/antib6040021. Antibodies (Basel). 2017. PMID: 31548537 Free PMC article.
-
Very high density of CHO cells in perfusion by ATF or TFF in WAVE bioreactor™. Part I. Effect of the cell density on the process.Biotechnol Prog. 2013 May-Jun;29(3):754-67. doi: 10.1002/btpr.1704. Epub 2013 May 21. Biotechnol Prog. 2013. PMID: 23436789 Free PMC article.
-
Particle movement and fluid behavior visualization using an optically transparent 3D-printed micro-hydrocyclone.Biomicrofluidics. 2020 Nov 19;14(6):064106. doi: 10.1063/5.0025391. eCollection 2020 Nov. Biomicrofluidics. 2020. PMID: 33269035 Free PMC article.
-
Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers.Tissue Eng Part A. 2012 Apr;18(7-8):806-15. doi: 10.1089/ten.TEA.2011.0391. Epub 2011 Dec 20. Tissue Eng Part A. 2012. PMID: 22011213 Free PMC article.
-
A novel technique for in situ aggregation of Gluconobacter oxydans using bio-adhesive magnetic nanoparticles.Biotechnol Bioeng. 2012 Dec;109(12):2970-7. doi: 10.1002/bit.24582. Epub 2012 Jul 12. Biotechnol Bioeng. 2012. PMID: 22729662 Free PMC article.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/bit.260450602', 'is_inner': False, 'url': 'https://doi.org/10.1002/bit.260450602'}, {'type': 'PubMed', 'value': '18623245', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/18623245/'}]}
- Al-Rubeai M, Singh RP, Goldman MH, Emery AN (1995a) Death mechanisms of animal cells in conditions of intensive agitation. Biotechnol Bioeng 45:463–472 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/bit.260460112', 'is_inner': False, 'url': 'https://doi.org/10.1002/bit.260460112'}, {'type': 'PubMed', 'value': '18623266', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/18623266/'}]}
- Al-Rubeai M, Singh RP, Emery AN, Zhang Z (1995b) Cell cycle and cell size dependence of susceptibility to hydrodynamic forces. Biotechnol Bioeng 46:88–92 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '9435462', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9435462/'}]}
- Al-Rubeai M (1998) Apoptosis and cell culture technology. Adv Biochem Eng Biotechnol 59:225–249 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1021/bp00006a600', 'is_inner': False, 'url': 'https://doi.org/10.1021/bp00006a600'}, {'type': 'PubMed', 'value': '1366836', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/1366836/'}]}
- Batt BC, Davis RH, Kompala DS (1990) Inclined sedimentation for selective retention of viable hybridomas in a continuous suspension bioreactor. Biotechnol Prog 6:458–464 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/bit.260400903', 'is_inner': False, 'url': 'https://doi.org/10.1002/bit.260400903'}, {'type': 'PubMed', 'value': '18601208', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/18601208/'}]}
- Born C, Zhang Z, Al-Rubeai M, Thomas CR (1992) Estimation of disruption of animal cells by laminar shear stress. Biotechnol Bioeng 40:1004–1010 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

