Development of a generic transient transfection process at 100 L scale
- PMID: 19002850
- PMCID: PMC2259262
- DOI: 10.1007/s10616-008-9135-2
Development of a generic transient transfection process at 100 L scale
Abstract
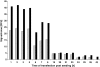
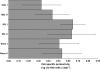
We have developed a generic transient transfection process at 100 L scale, using HEK293-EBNA cells and PEI as the transfection reagent for the production of recombinant IgG. The process, including large-scale plasmid preparation, expression at bioreactor scale, capture, purification and, if necessary, endotoxin removal allows reproducible production of more than 0.5 g IgG for in vitro and in vivo studies. We compared the performance of two HEK cell lines, investigated the effect of conditioned medium, optimized the DNA:PEI ratio and implemented a feed strategy to prolong the culture time to increase product yield. The transient transfection protocol developed enables a closed process from seeding culture to protein capture. The challenge of performing a medium exchange before transfection at large scale is solved by applying a continuous centrifugation step between the seeding bioreactor and the production bioreactor. After 7-8 days the harvest and capture is performed in a one-step operation using a Streamline expanded bed chromatography system. Following a polishing step the purified antibody is transferred to the final formulation buffer. The method has shown to be reproducible at 10, 50, and 100 L scale expressing between 5 and 8 mg L(-1) IgG.
Figures




Similar articles
-
Transient gene expression in mammalian cells grown in serum-free suspension culture.Cytotechnology. 1999 Jul;30(1-3):71-83. doi: 10.1023/A:1008000327766. Cytotechnology. 1999. PMID: 19003357 Free PMC article.
-
Transient gene expression: recombinant protein production with suspension-adapted HEK293-EBNA cells.Biotechnol Bioeng. 2001 Oct 20;75(2):197-203. doi: 10.1002/bit.1179. Biotechnol Bioeng. 2001. PMID: 11536142
-
A high cell density transient transfection system for therapeutic protein expression based on a CHO GS-knockout cell line: process development and product quality assessment.Biotechnol Bioeng. 2015 May;112(5):977-86. doi: 10.1002/bit.25514. Epub 2015 Mar 16. Biotechnol Bioeng. 2015. PMID: 25502369
-
High-density transient gene expression in suspension-adapted 293 EBNA1 cells.Biotechnol Bioeng. 2008 Jan 1;99(1):108-16. doi: 10.1002/bit.21537. Biotechnol Bioeng. 2008. PMID: 17630648
-
Plasmid DNA purification.J Gene Med. 2004 Feb;6 Suppl 1:S54-66. doi: 10.1002/jgm.512. J Gene Med. 2004. PMID: 14978751 Review.
Cited by
-
Expression of recombinant antibodies.Front Immunol. 2013 Jul 29;4:217. doi: 10.3389/fimmu.2013.00217. eCollection 2013. Front Immunol. 2013. PMID: 23908655 Free PMC article.
-
The transient expression of CHIKV VLP in large stirred tank bioreactors.Cytotechnology. 2019 Dec;71(6):1079-1093. doi: 10.1007/s10616-019-00346-x. Epub 2019 Sep 27. Cytotechnology. 2019. PMID: 31560090 Free PMC article.
-
Better and faster: improvements and optimization for mammalian recombinant protein production.Curr Opin Struct Biol. 2014 Jun;26:39-43. doi: 10.1016/j.sbi.2014.03.006. Epub 2014 Apr 12. Curr Opin Struct Biol. 2014. PMID: 24721463 Free PMC article. Review.
-
An anti-apoptotic HEK293 cell line provides a robust and high titer platform for transient protein expression in bioreactors.MAbs. 2019 Jul;11(5):977-986. doi: 10.1080/19420862.2019.1598230. Epub 2019 Apr 24. MAbs. 2019. PMID: 30907238 Free PMC article.
-
Lentiviral Vector Bioprocessing.Viruses. 2021 Feb 9;13(2):268. doi: 10.3390/v13020268. Viruses. 2021. PMID: 33572347 Free PMC article. Review.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/j.jchromb.2006.09.026', 'is_inner': False, 'url': 'https://doi.org/10.1016/j.jchromb.2006.09.026'}, {'type': 'PubMed', 'value': '17046339', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17046339/'}]}
- Abhinav A, Shukla A, Hubbard B, Tressel T et al (2007) Downstream processing of monoclonal antibodies—Application of platform approaches. J Chromatogr 848:28–39 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1021/bp049830x', 'is_inner': False, 'url': 'https://doi.org/10.1021/bp049830x'}, {'type': 'PubMed', 'value': '15903252', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15903252/'}]}
- Baldi L, Muller N, Picasso S et al (2005) Transient gene expression in suspension HEK-293 cells: application to large-scale protein production. Biotechnol Prog 21:148–153 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1074/jbc.274.27.19375', 'is_inner': False, 'url': 'https://doi.org/10.1074/jbc.274.27.19375'}, {'type': 'PubMed', 'value': '10383450', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10383450/'}]}
- Belting M, Petersson P (1999) Intracellular accumulation of secreted proteoglycans inhibits cationic lipid-mediated gene transfer. Co-transfer of glycosaminoglycans to the nucleus. J Biol Chem 274(27):19375–19382 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/j.addr.2004.10.004', 'is_inner': False, 'url': 'https://doi.org/10.1016/j.addr.2004.10.004'}, {'type': 'PubMed', 'value': '15722161', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15722161/'}]}
- Belting M, Sandgren S, Wittrup A (2005) Nuclear delivery of macromolecules: barriers and carriers. Adv Drug Deliv Rev 57(4):505–527 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '16082021', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16082021/'}]}
- Birch J, Onakunle Y (2005) Biopharmaceutical proteins: opportunities and challenges. In: Smales CM, James DC (eds) Therapeutic proteins: methods and protocols. Totowa, Humana Press, pp 1–16 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

