Small interfering RNA knockdown of mini-TyrRS and mini-TrpRS effects angiogenesis in human umbilical vein endothelial cells in hypoxic culture
- PMID: 19002860
- PMCID: PMC2553632
- DOI: 10.1007/s10616-008-9151-2
Small interfering RNA knockdown of mini-TyrRS and mini-TrpRS effects angiogenesis in human umbilical vein endothelial cells in hypoxic culture
Abstract
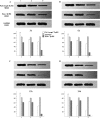
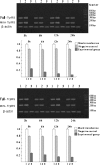
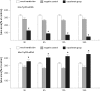
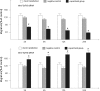
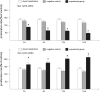
Aim We studied the role of mini-TyrRS and mini-TrpRS in angiogenesis by using small interfering RNA-mediated mini-TyrRS/mini-TrpRS knockout in hypoxic culture of human umbilical vein endothelial cells. Methods SiRNA was used as the main method to inhibited the gene function. Silencing efficiency was assayed by real-time reverse transcription-polymerase chain reaction and western blotting. The angiogenic activity in vitro was evaluated by transwell migration assay and Matrigel-induced capillary tube formation in hypoxic culture. Cell proliferation was determined by crystal violet staining. Results The results showed that levels of the mini-TyrRS/mini-TrpRS gene and protein in mock transfection group and negative control group were higher, but noticeably decreased in experimental group. However, no significant difference was detected between mock transfection group and negative control group, but there was a statistically significant difference compared with experimental group. For mini-TyrRS-siRNA group, the cell migration, tube formation and the rate of cell proliferation were respectively inhibited by (47.4, 56.3, 65.4, 73.7%), (60.5, 69.1, 75.9, 83.6%) and (40.4, 56.2, 61.2, 68.0%). For mini-TrpRS-siRNA, were respectively increased by (18.0, 33.8, 45.1, 56.4%), (18.3, 31.2, 40.3, 45.7%) and (8.4, 26.4, 38.2, 46.6%). Conclusion These results indicated that angiogenesis is either stimulated by mini-TyrRS or inhibited by mini-TrpRS in matrigel models in hypoxic culture, raising the possibility that mini-TyrRS stimulates a common downstream signaling event. Thus, naturally occurring fragments of two proteins involved in translation, TyrRS and TrpRS, have opposing activity on endothelial cell angiogenesis in the matrigel assays. The opposing activities of the two tRNA synthetases suggest tight regulation of the balance between pro- and anti-angiogenic stimuli.
Figures



Similar articles
-
Different angiogenesis effect of mini-TyrRS/mini-TrpRS by systemic administration of modified siRNAs in rats with acute myocardial infarction.Heart Vessels. 2010 Jul;25(4):324-32. doi: 10.1007/s00380-009-1200-z. Epub 2010 Jul 31. Heart Vessels. 2010. PMID: 20676842
-
Inhibition of mini-TyrRS-induced angiogenesis response in endothelial cells by VE-cadherin-dependent mini-TrpRS.Heart Vessels. 2012 Mar;27(2):193-201. doi: 10.1007/s00380-011-0137-1. Epub 2011 Mar 26. Heart Vessels. 2012. PMID: 21442253
-
VEGF, not VEGFR2, is associated with the angiogenesis effect of mini-TyrRS/mini-TrpRS in human umbilical vein endothelial cells in hypoxia.Cytotechnology. 2014 Aug;66(4):655-65. doi: 10.1007/s10616-013-9619-6. Epub 2013 Jul 30. Cytotechnology. 2014. PMID: 23896703 Free PMC article.
-
Inhibition of tumor angiogenesis by a natural fragment of a tRNA synthetase.Trends Biochem Sci. 2006 Jan;31(1):7-10. doi: 10.1016/j.tibs.2005.11.002. Epub 2005 Nov 16. Trends Biochem Sci. 2006. PMID: 16297628 Review.
-
Non-canonical functions of human cytoplasmic tyrosyl-, tryptophanyl- and other aminoacyl-tRNA synthetases.Enzymes. 2020;48:207-242. doi: 10.1016/bs.enz.2020.04.001. Epub 2020 Jun 12. Enzymes. 2020. PMID: 33837705 Review.
Cited by
-
Different angiogenesis effect of mini-TyrRS/mini-TrpRS by systemic administration of modified siRNAs in rats with acute myocardial infarction.Heart Vessels. 2010 Jul;25(4):324-32. doi: 10.1007/s00380-009-1200-z. Epub 2010 Jul 31. Heart Vessels. 2010. PMID: 20676842
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '11910072', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11910072/'}]}
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Sciences (New York) 296:550–553 - PubMed
-
- None
- Byrom M, Pallotta V, Brown D et al (2002) Visualizing SiRNA in mammalian cells: fluorescence analysis of the RNAi effect. Ambion Technotes 9(3):6–8
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1073/pnas.90.8.3574', 'is_inner': False, 'url': 'https://doi.org/10.1073/pnas.90.8.3574'}, {'type': 'PMC', 'value': 'PMC46343', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC46343/'}, {'type': 'PubMed', 'value': '8475106', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8475106/'}]}
- Clark LI, Dewald B, Geiser T et al (1993) Platelet factor 4 binds to interleukin 8 receptors and activates neutrophils when its N terminus is modified with Glu-Leu-Arg. Proc Natl Acad Sci USA 90:3574–3577. doi:10.1073/pnas.90.8.3574 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/35078107', 'is_inner': False, 'url': 'https://doi.org/10.1038/35078107'}, {'type': 'PubMed', 'value': '11373684', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11373684/'}]}
- Elbashir SM, Harborth J, Lendeckel W et al (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in Drosophila melanogaster embryo lysate. Nature 411:494–498. doi:10.1038/35078107 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1021/bi020537k', 'is_inner': False, 'url': 'https://doi.org/10.1021/bi020537k'}, {'type': 'PubMed', 'value': '12416978', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12416978/'}]}
- Ewalt KL, Schimmel P (2002) Activation of angiogenic signaling pathways by two human tRNA synthetases. Biochemistry 41:13344–13349. doi:10.1021/bi020537k - PubMed
LinkOut - more resources
Full Text Sources

