Cell cycle phase dependent productivity of a recombinant Chinese hamster ovary cell line
- PMID: 19002865
- PMCID: PMC3449425
- DOI: 10.1007/s10616-006-9041-4
Cell cycle phase dependent productivity of a recombinant Chinese hamster ovary cell line
Abstract
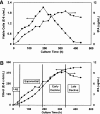
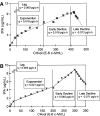
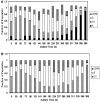
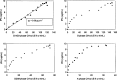
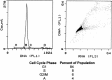
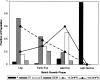
A Chinese Hamster Ovary cell line, CHO1-15(500), producing recombinant human tissue type plasminogen activator (tPA) via the dihydrofolate reductase (DHFR) amplification system, was studied in batch culture. In this system both DHFR and tPA are under the control of the strong constitutive viral SV40 early promoter. Employing the cumulative viable cell-hour approach, the specific productivity of tPA had maxima in the lag (0.065 pg cell(-1 )h(-1)) and early decline (0.040 pg cell(-1 )h(-1)) population growth phases. The viable population was assigned into four subpopulations (G1, S, G2/M phase, and Apoptotic cells) using flow cytometric analysis. As expected, intracellular DHFR was maximally expressed during the S cell cycle phase. The production of tPA, however, was found to be a direct linear function of the G1 phase, with a subpopulation specific productivity of 0.080 pg c-h(-1). Productivity maxima in the lag and early decline corroborate the flow cytometric data, indicative that this recombinant tPA production occurs primarily in the G1 cell cycle phase, not the S phase. This suggests that endogenous regulatory mechanisms are important controlling influences on the production of recombinant tPA in this ovarian cell line. Productivity from recombinant cell lines cannot be inferred from either the plasmid construct or the host cell alone. Elucidation of the relationship between expression of recombinant protein and the cell cycle phases of the host cell is a major component of the characterization of the animal cell production system. This information facilitates rational process design, including operating mode, modelling and control, and medium formulation.
Figures
Similar articles
-
Cell cycle-dependent DHFR and t-PA production in cotransfected, MTX-amplified CHO cells revealed by dual-laser flow cytometry.Exp Cell Res. 1990 Jun;188(2):267-71. doi: 10.1016/0014-4827(90)90169-b. Exp Cell Res. 1990. PMID: 2110526
-
Generation of recombinant CHO(dhfr-) cell lines by single selection for dhfr+ transformants.Anal Biochem. 1992 May 15;203(1):146-50. doi: 10.1016/0003-2697(92)90055-c. Anal Biochem. 1992. PMID: 1524211
-
High glucose and low specific cell growth but not mild hypothermia improve specific r-protein productivity in chemostat culture of CHO cells.PLoS One. 2018 Aug 16;13(8):e0202098. doi: 10.1371/journal.pone.0202098. eCollection 2018. PLoS One. 2018. PMID: 30114204 Free PMC article.
-
Effect of Vitreoscilla hemoglobin expression on growth and specific tissue plasminogen activator productivity in recombinant chinese hamster ovary cells.Biotechnol Bioeng. 1994 Dec;44(11):1367-70. doi: 10.1002/bit.260441114. Biotechnol Bioeng. 1994. PMID: 18618650
-
Appropriate mammalian expression systems for biopharmaceuticals.Arzneimittelforschung. 1998 Aug;48(8):870-80. Arzneimittelforschung. 1998. PMID: 9748718 Review.
Cited by
-
Optimum blue light exposure: a means to increase cell-specific productivity in Chinese hamster ovary cells.Appl Microbiol Biotechnol. 2024 Dec 5;108(1):530. doi: 10.1007/s00253-024-13363-4. Appl Microbiol Biotechnol. 2024. PMID: 39636393 Free PMC article.
-
Mapping the molecular basis for growth related phenotypes in industrial producer CHO cell lines using differential proteomic analysis.BMC Biotechnol. 2021 Jul 23;21(1):43. doi: 10.1186/s12896-021-00704-8. BMC Biotechnol. 2021. PMID: 34301236 Free PMC article.
-
SWATH-MS insights on sodium butyrate effect on mAbs production and redox homeostasis in CHO cells.AMB Express. 2024 Dec 24;14(1):140. doi: 10.1186/s13568-024-01807-z. AMB Express. 2024. PMID: 39718710 Free PMC article.
-
Transferability of miRNA-technology to bioprocessing: Influence of cultivation mode and media.Biotechnol Prog. 2021 Mar;37(2):e3107. doi: 10.1002/btpr.3107. Epub 2020 Dec 30. Biotechnol Prog. 2021. PMID: 33300297 Free PMC article.
-
Enhanced recombinant factor VII expression in Chinese hamster ovary cells by optimizing signal peptides and fed-batch medium.Bioengineered. 2016 Apr;7(3):189-97. doi: 10.1080/21655979.2016.1176656. Epub 2016 Apr 26. Bioengineered. 2016. PMID: 27116572 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

