Anoikis-resistant MDCK cells carrying susceptibilities to TNF-alpha and verotoxin that are suitable for influenza virus cultivation
- PMID: 19002866
- PMCID: PMC3449419
- DOI: 10.1007/s10616-006-9032-5
Anoikis-resistant MDCK cells carrying susceptibilities to TNF-alpha and verotoxin that are suitable for influenza virus cultivation
Abstract
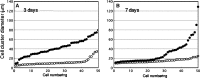
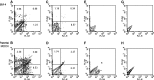
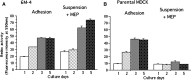
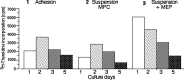
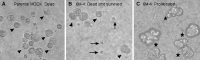
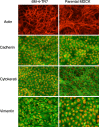
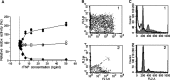
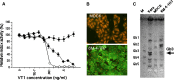
Madin-Darby canine kidney (MDCK) cells were originally anchorage-dependent epithelial cells. Here, we have isolated a novel MDCK-derived cell population, termed 6 M-4, by means of culturing MDCK cells in suspension for nearly 6 months in the presence of Streptomyces griseus metalloendopeptidase (MEP). The isolated cells showed unique proliferation characteristics, which differed from parental MDCK cells. They proliferated adherently on a polystyrene matrix, but proliferated non-adherently both in the presence of MEP and on a non-adhesive matrix coated with poly 2-methacryloyloxyethyl phosphorylcholine (MPC). The 6 M-4 cells consisted of at least two cell types. One type, termed 6 M-4-TR7, would not grow in soft agar and showed a novel phenotype in that the cells were susceptible to both TNF-alpha and verotoxin 1 (VT1). In addition, the isolated adhesion-independent cells sustained epithelial traits of parental MDCK cells. We further show that these MDCK-derivative cells are suitable for influenza virus cultivation. Hemagglutination (HA) titers of influenzaviruses A and B were increased in the suspension culture of 6 M-4-TR7 cells supplemented with the MEP in comparison to adherently growing cells in the presence of trypsin.
Figures


Similar articles
-
Comparison of influenza virus yields and apoptosis-induction in an adherent and a suspension MDCK cell line.Vaccine. 2013 Nov 19;31(48):5693-9. doi: 10.1016/j.vaccine.2013.09.051. Epub 2013 Oct 8. Vaccine. 2013. PMID: 24113260
-
Comparison of immunogenicity of cell-and egg-passaged viruses for manufacturing MDCK cell culture-based influenza vaccines.Virus Res. 2015 Jun 2;204:40-6. doi: 10.1016/j.virusres.2015.04.005. Epub 2015 Apr 17. Virus Res. 2015. PMID: 25892718
-
Systematic evaluation of suspension MDCK cells, adherent MDCK cells, and LLC-MK2 cells for preparing influenza vaccine seed virus.Vaccine. 2019 Oct 8;37(43):6526-6534. doi: 10.1016/j.vaccine.2019.08.064. Epub 2019 Sep 6. Vaccine. 2019. PMID: 31500967
-
Conversion of MDCK cell line to suspension culture by transfecting with human siat7e gene and its application for influenza virus production.Proc Natl Acad Sci U S A. 2009 Sep 1;106(35):14802-7. doi: 10.1073/pnas.0905912106. Epub 2009 Aug 17. Proc Natl Acad Sci U S A. 2009. PMID: 19706449 Free PMC article.
-
Adaptation of a Madin-Darby canine kidney cell line to suspension growth in serum-free media and comparison of its ability to produce avian influenza virus to Vero and BHK21 cell lines.J Virol Methods. 2011 Jan;171(1):53-60. doi: 10.1016/j.jviromet.2010.09.029. Epub 2010 Oct 7. J Virol Methods. 2011. PMID: 20933017
Cited by
-
Characterization and Immunogenicity of Influenza H7N9 Vaccine Antigens Produced Using a Serum-Free Suspension MDCK Cell-Based Platform.Viruses. 2022 Aug 31;14(9):1937. doi: 10.3390/v14091937. Viruses. 2022. PMID: 36146744 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

