Isolation of progenitor cells from cord blood using adhesion matrices
- PMID: 19002871
- PMCID: PMC3449415
- DOI: 10.1007/s10616-007-9043-x
Isolation of progenitor cells from cord blood using adhesion matrices
Erratum in
-
Isolation of progenitor cells from cord blood using adhesion matrices.Cytotechnology. 2007 Jun;54(2):121-33. doi: 10.1007/s10616-007-9077-0. Epub 2007 Jun 30. Cytotechnology. 2007. PMID: 19003027 Free PMC article.
Abstract
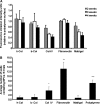
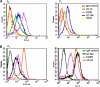
The aim of this study was to develop optimal conditions for selective adhesion and isolation of mesenchymal progenitor cells (PCs) from cord blood and to determine their potential for osteogenic differentiation. Mononuclear cells (MNCs) were isolated by Ficoll-Paque gradient and plated onto 48-well culture plates precoated with: human or bovine collagen type I, human collagen type IV, fibronectin or matrigel. Cultures were incubated in alphaMEM containing fetal calf serum. Viability of the adherent cells was determined by alamarBlue(R) assay after 2, 3, and 4 weeks. After 4 weeks in culture, cells were typsinized and replated. Primary cultures were analyzed by histochemistry and third passage cells by FACS. Isolated fibroblast-like cells were cultured in the presence of osteogenic factors and differentiation determined by Alizarin Red S staining, RT-PCR and electron dispersive spectroscopy (EDS). MNCs adhered to all types of matrices with the greatest adhesion rates on fibronectin. These cells were CD45(+), CD105(+), CD14(+), CD49a(+), CD49f(+), CD44(+) and CD34(-). The highest incidence of PCs was observed on fibronectin and polystyrene. Passages were CD45(-), CD14(-), CD34(-) and weakly CD105(+). Primary cultures expressed endothelial/macrophage RNA markers whether cultured on fibronectin or polystyrene and these markers decreased upon passage. The best osteogenic differentiation was observed in MPCs cultured in osteogenic medium containing Vit D(3) and FGF9. These cells expressed the bone-related mRNA, collagen type I, core binding factor I (Cbfa I), osteocalcin and osteopontin. EDS of deposits produced by these cells demonstrated a calcium/phosphate ratio parallel to hydroxyapatite. It was concluded that fibronectin increased adhesion rates and isolation potential of cord blood mesenchymal progenitor cells.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

