pH Condition in temperature shift cultivation enhances cell longevity and specific hMab productivity in CHO culture
- PMID: 19002878
- PMCID: PMC3449411
- DOI: 10.1007/s10616-007-9059-2
pH Condition in temperature shift cultivation enhances cell longevity and specific hMab productivity in CHO culture
Abstract
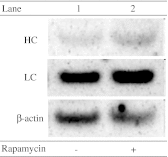
Controlling cell proliferation during cell culturing is an effective way to improve the production yield in mammalian cell culture. We examined the effect of temperature shifts (TS) under pH control conditions in Chinese hamster ovary cells. When we shifted the culture temperature from 37 degrees C to 31 degrees C before a stationary phase at pH 6.8 (TS/pH 6.8), cell viability remained high, and the final human monoclonal antibody (hMab) concentration increased to 2.3 times that in the culture remaining at 37 degrees C. However, there were no significant effects on the cell viability or production yield with the same TS at pH 7.0 (TS/pH 7.0). The average specific hMab productivity and mRNA level of TS/pH 7.0 were the same as that of TS/pH 6.8. The control of cell growth by the TS or the addition of rapamycin was effective in the maintenance of cell viability, but there was no significant increase of the average specific hMab productivity in the culture where cell proliferation was controlled with rapamycin. The hMab mRNA concentration decreased to 55%-65% at a 37 degrees C culture with the addition of actinomycin D. In contrast, actinomycin D did not affect the mRNA level in the TS culture. This result suggested that the increase in the mRNA level in the TS condition was caused by an increase in mRNA stability. In this study, we show that TS can produce two unrelated effects: a prolongation of cell longevity and an improvement in mRNA stability.
Figures



References
-
- Giard DJ, Fleischaker RJ, Fabricant M. Effect of temperature on the production of human fibroblast interferon. Proc Soc Exp Biol Med. 1982;170:155–159. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

