Intracellular nucleotide pools and ratios as tools for monitoring dedifferentiation of primary porcine hepatocytes in culture
- PMID: 19002882
- PMCID: PMC3449807
- DOI: 10.1007/s10616-006-9019-2
Intracellular nucleotide pools and ratios as tools for monitoring dedifferentiation of primary porcine hepatocytes in culture
Abstract
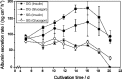
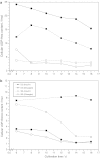
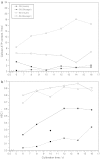
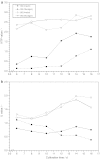
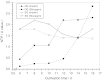
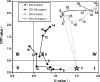
The effect of two culture configurations (single collagen gel and double collagen gel) and of two hormones (insulin and glucagon) on the differentiated status and the intracellular nucleotide pools of primary porcine hepatocytes was investigated. The objective was to analyze and monitor the current state of differentiation supported by the two culture modes using intracellular nucleotide analysis. Specific intracellular nucleotide ratios, namely the nucleoside triphosphate (NTP) and the uridine (U) ratio were shown to consistently reflect the state of dedifferentiation status of the primary cells in culture affected by the presence of the two hormones insulin and glucagon. Continuous dedifferentiation of the cells was monitored in parallel by the reduction of the secretion of albumin, and changes in UDP-activated hexoses and UDP-glucuronate. The presence of insulin maintained the differentiated status of hepatocytes for more than 12 days when cultivated under double gel conditions whereas glucagon was less effective. In contrast, cells cultivated in a single gel matrix immediately started to dedifferentiate upon seeding. NTP and U ratios were shown to be more sensitive for monitoring dedifferentiation in culture than the albumin secretion. Their use allowed the generation of an easily applicable NTP-U plot in order to give a direct graphical representation of the current differentiation status of the cultured cells. Moreover, the transition from functional and differentiated hepatocytes to dedifferentiated fibroblasts could be determined earlier by the nucleotide ratios compared to the conventional method of monitoring the albumin secretion rate.
Figures

Similar articles
-
Intracellular ribonucleotide pools as a tool for monitoring the physiological state of in vitro cultivated mammalian cells during production processes.Biotechnol Bioeng. 1992 Oct 20;40(8):934-46. doi: 10.1002/bit.260400810. Biotechnol Bioeng. 1992. PMID: 18601201
-
Collagen sandwich culture affects intracellular polyamine levels of human hepatocytes.Cell Prolif. 2002 Oct;35(5):257-67. doi: 10.1046/j.1365-2184.2002.00248.x. Cell Prolif. 2002. PMID: 12269903 Free PMC article.
-
Extracellular matrix modulates sensitivity of hepatocytes to fibroblastoid dedifferentiation and transforming growth factor beta-induced apoptosis.Hepatology. 2009 Jun;49(6):2031-43. doi: 10.1002/hep.22880. Hepatology. 2009. PMID: 19274752
-
Molecular mechanisms underlying the dedifferentiation process of isolated hepatocytes and their cultures.Curr Drug Metab. 2006 Aug;7(6):629-60. doi: 10.2174/138920006778017759. Curr Drug Metab. 2006. PMID: 16918317 Review.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
Cited by
-
Culture of primary bovine chondrocytes on a continuously expanding surface inhibits dedifferentiation.Tissue Eng Part A. 2012 Dec;18(23-24):2466-76. doi: 10.1089/ten.TEA.2012.0215. Epub 2012 Aug 3. Tissue Eng Part A. 2012. PMID: 22738340 Free PMC article.
References
-
- Atkinson DA. Cellular energy metabolism and its regulation. New York: Academic Press; 1977.
-
- Andus T, Geiger T, Hirano T, Kishimoto T, Tran-thi TA, Decker K, Heinrich PC. Regulation of synthesis and secretion of major acute-phase proteins by recombinant human interleukin-6 (BSF-2/IL-6) in hepatocyte primary culture. Eur J Biochem. 1988;173:287–293. doi: 10.1111/j.1432-1033.1988.tb13997.x. - DOI - PubMed
-
- Annoni G, Weiner FR, Colombo M, Czaja MJ, Zern MA. Albumin and collagen gene regulation in alcohol- and virus-induced human liver. Gastroenterology. 1990;98:197–202. - PubMed
-
- Bader A, Bartolo L, Haverich A. Initial evaluation of the performance of a scaled-up flat membrane bioreactor (FMB) with pig liver cells. In: Crepaldi G, Demetrioou AA, Muraca M, editors. Bioartificial liver: the critical issues. Rome: CIC International Editions; 1997. pp. 36–41.
LinkOut - more resources
Full Text Sources

