A serum-free Vero production platform for a chimeric virus vaccine candidate
- PMID: 19002888
- PMCID: PMC3449809
- DOI: 10.1007/s10616-006-9030-7
A serum-free Vero production platform for a chimeric virus vaccine candidate
Abstract
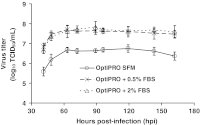
MedImmune Vaccines has engineered a live, attenuated chimeric virus that could prevent infections caused by parainfluenza virus type 3 (PIV3) and respiratory syncytial virus (RSV), causative agents of acute respiratory diseases in infants and young children. The work here details the development of a serum-free Vero cell culture production platform for this virus vaccine candidate. Efforts to identify critical process parameters and optimize culture conditions increased infectious virus titers by approximately 2 log(10) TCID(50)/ml over the original serum-free process. In particular, the addition of a chemically defined lipid concentrate to the pre-infection medium along with the shift to a lower post-infection cultivation temperature increased virus titers by almost 100-fold. This improved serum-free process achieved comparable virus titers to the serum-supplemented process, and demonstrated consistent results upon scale-up: Vero cultures in roller bottles, spinner flasks and bioreactors reproducibly generated maximum infectious virus titers of 8 log(10) TCID(50)/ml.
Figures



Similar articles
-
Packaging and Prefusion Stabilization Separately and Additively Increase the Quantity and Quality of Respiratory Syncytial Virus (RSV)-Neutralizing Antibodies Induced by an RSV Fusion Protein Expressed by a Parainfluenza Virus Vector.J Virol. 2016 Oct 14;90(21):10022-10038. doi: 10.1128/JVI.01196-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27581977 Free PMC article.
-
Enhanced Neutralizing Antibody Response Induced by Respiratory Syncytial Virus Prefusion F Protein Expressed by a Vaccine Candidate.J Virol. 2015 Sep;89(18):9499-510. doi: 10.1128/JVI.01373-15. Epub 2015 Jul 8. J Virol. 2015. PMID: 26157122 Free PMC article.
-
Attenuated Human Parainfluenza Virus Type 1 Expressing the Respiratory Syncytial Virus (RSV) Fusion (F) Glycoprotein from an Added Gene: Effects of Prefusion Stabilization and Packaging of RSV F.J Virol. 2017 Oct 27;91(22):e01101-17. doi: 10.1128/JVI.01101-17. Print 2017 Nov 15. J Virol. 2017. PMID: 28835504 Free PMC article.
-
Medical burden of respiratory syncytial virus and parainfluenza virus type 3 infection among US children. Implications for design of vaccine trials.Hum Vaccin. 2005 Jan-Feb;1(1):6-11. doi: 10.4161/hv.1.1.1424. Epub 2005 Jan 10. Hum Vaccin. 2005. PMID: 17038832 Review.
-
Current status of vaccines for parainfluenza virus infections.Pediatr Infect Dis J. 2008 Oct;27(10 Suppl):S123-5. doi: 10.1097/INF.0b013e318168b76f. Pediatr Infect Dis J. 2008. PMID: 18820572 Review.
Cited by
-
Development of an animal component free production process for Sabin inactivated polio vaccine.Vaccine X. 2022 Sep 30;12:100223. doi: 10.1016/j.jvacx.2022.100223. eCollection 2022 Dec. Vaccine X. 2022. PMID: 36217423 Free PMC article.
-
High-titer manufacturing of SARS-CoV-2 Spike-pseudotyped VSV in stirred-tank bioreactors.Mol Ther Methods Clin Dev. 2024 Jan 17;32(1):101189. doi: 10.1016/j.omtm.2024.101189. eCollection 2024 Mar 14. Mol Ther Methods Clin Dev. 2024. PMID: 38327804 Free PMC article.
-
Challenges and Opportunities in the Process Development of Chimeric Vaccines.Vaccines (Basel). 2023 Dec 8;11(12):1828. doi: 10.3390/vaccines11121828. Vaccines (Basel). 2023. PMID: 38140232 Free PMC article. Review.
-
Suspension culture of Vero cells for the production of adenovirus type 5.Clin Exp Vaccine Res. 2020 Jan;9(1):48-55. doi: 10.7774/cevr.2020.9.1.48. Epub 2020 Jan 31. Clin Exp Vaccine Res. 2020. PMID: 32095440 Free PMC article.
-
A single amino acid in the F2 subunit of respiratory syncytial virus fusion protein alters growth and fusogenicity.J Gen Virol. 2013 Dec;94(Pt 12):2627-2635. doi: 10.1099/vir.0.055368-0. Epub 2013 Oct 3. J Gen Virol. 2013. PMID: 24092758 Free PMC article.
References
-
- Asher DM. The transmissible spongiform encephalopathy agents: concerns and responses of United States regulatory agencies in maintaining the safety of biologics. Dev Biol Stand. 1999;100:103–118. - PubMed
-
- Brown G, Rixon HWM, Steel J, McDonald TP, Pitt AR, Graham S, Sugrue RJ. Evidence for an association between heat shock protein 70 and the respiratory syncytial virus polymerase complex within lipid-raft membranes during virus infection. Virology. 2005;338:69–80. doi: 10.1016/j.virol.2005.05.004. - DOI - PubMed
-
- Castle P, Robertson JS. Animal sera, animal sera derivatives and substitutes used in the manufacture of pharmaceuticals: viral safety and regulatory aspects. Dev Biol Stand. 1999;99:191–196. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

