On-line monitoring of infected Sf-9 insect cell cultures by scanning permittivity measurements and comparison with off-line biovolume measurements
- PMID: 19003001
- PMCID: PMC2104552
- DOI: 10.1007/s10616-007-9093-0
On-line monitoring of infected Sf-9 insect cell cultures by scanning permittivity measurements and comparison with off-line biovolume measurements
Abstract
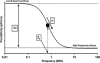
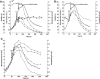
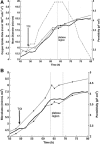
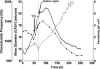
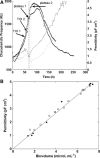
Two infected Sf-9 cell cultures were monitored on-line by multi-frequency permittivity measurements using the Fogale BIOMASS SYSTEM((R)) and by applying different off-line methods (CASY((R))1, Vi-CELLtrade mark, packed cell volume) to measure the biovolume and the mean diameter of the cell population. During the growth phase and the early infection phase the measured permittivity at the working frequency correlated well with the different off-line methods for the biovolume. We found a value of 0.67 pF cm(-1) permittivity per unit of total biovolume (CASY) (muL mL(-1)). After the maximum value in the permittivity was reached, i.e. when the viability of the cultures decreased significantly, we observed different time courses for the biovolume depending on the applied method. The differences were compared and could be explained by the underlying measurement principles. Furthermore, the characteristic frequency (f(C)) was calculated from the on-line scanning permittivity measurements. The f(C) may provide an indication of changes in cell diameter and membrane properties especially after infection and could also be an indicator for the onset of the virus production phase. The changes in f(C) were qualitatively explained by the underlying equation that is correlating f(C) and the properties of the cell population (cell diameter, intracellular conductivity and capacitance per membrane area).
Figures
References
-
- Ansorge S, Esteban G, Ghommidh C, Schmid G (2007) Monitoring nutrient limitations by online capactitance measurements in batch and fed-batch CHO fermentations. In: Smith R (ed) Conference Proceedings to the 19th ESACT Meeting: Cell Technology for Cell Products. Springer, Dordrecht/NL, pp 723–726
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/bit.10809', 'is_inner': False, 'url': 'https://doi.org/10.1002/bit.10809'}, {'type': 'PubMed', 'value': '14574694', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/14574694/'}]}
- Cannizzaro C, Gugerli R, Marison I, von Stockar U (2003) On-line biomass monitoring of CHO perfusion culture with scanning dielectric spectroscopy. Biotechnol Bioeng 84:597–610 - PubMed
-
- None
- Chico E, Jäger V (1998) Measurements of changes in cell size distribution to monitor Baculovirus infection of insect cells. In: Merten OW, Perrin P, Griffiths B (eds) New developments and new applications in animal cell technology. Kluwer Academic Publishers, Dordrecht, 329–331
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/0003-2697(89)90191-7', 'is_inner': False, 'url': 'https://doi.org/10.1016/0003-2697(89)90191-7'}, {'type': 'PubMed', 'value': '2667390', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/2667390/'}]}
- Cook JA, Mitchell JB (1989) Viability measurements in mammalian cell systems. Anal Biochem 179:1–7 - PubMed
-
- None
- Davey CL (1993) The biomass monitor source book. Department of Biological Sciences, University of Wales, Aberystwyth
LinkOut - more resources
Full Text Sources
Other Literature Sources

