Three-dimensional reconstruction of the amphid sensilla in the microbial feeding nematode, Acrobeles complexus (Nematoda: Rhabditida)
- PMID: 19003904
- PMCID: PMC2750866
- DOI: 10.1002/cne.21882
Three-dimensional reconstruction of the amphid sensilla in the microbial feeding nematode, Acrobeles complexus (Nematoda: Rhabditida)
Abstract
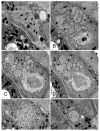
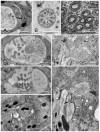
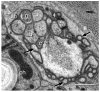
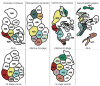
Amphid sensilla are the primary olfactory, chemoreceptive, and thermoreceptive organs in nematodes. Their function is well described for the model organism Caenorhabditis elegans, but it is not clear to what extent we can generalize these findings to distantly related nematodes of medical, economic, and agricultural importance. Current detailed descriptions of anatomy and sensory function are limited to nematodes that recent molecular phylogenies would place in the same taxonomic family, the Rhabditidae. Using serial thin-section transmission electron microscopy, we reconstructed the anatomy of the amphid sensilla in the more distantly related nematode, Acrobeles complexus (Cephalobidae). Amphid structure is broadly conserved in number and arrangement of cells. Details of cell anatomy differ, particularly for the sensory neurite termini. We identify an additional sensory neuron not found in the amphid of C. elegans and propose homology with the C. elegans interneuron AUA. Hypotheses of homology for the remaining sensory neurons are also proposed based on comparisons between C. elegans, Strongyloides stercoralis, and Haemonchus contortus.
Figures


Similar articles
-
Resolving phylogenetic incongruence to articulate homology and phenotypic evolution: a case study from Nematoda.Proc Biol Sci. 2010 May 7;277(1686):1299-307. doi: 10.1098/rspb.2009.2195. Epub 2010 Jan 27. Proc Biol Sci. 2010. PMID: 20106846 Free PMC article. Review.
-
Three-dimensional reconstruction of the nose epidermal cells in the microbial feeding nematode, Acrobeles complexus (Nematoda: Rhabditida).J Morphol. 2006 Nov;267(11):1257-72. doi: 10.1002/jmor.10456. J Morphol. 2006. PMID: 16710857
-
Three-dimensional fine structural reconstruction of the nose sensory structures of Acrobeles complexus compared to Caenorhabditis elegans (Nematoda: Rhabditida).J Morphol. 2007 Aug;268(8):649-63. doi: 10.1002/jmor.10535. J Morphol. 2007. PMID: 17514723
-
Comparative, three-dimensional anterior sensory reconstruction of Aphelenchus avenae (nematoda: Tylenchomorpha).J Comp Neurol. 2009 Dec 10;517(5):616-32. doi: 10.1002/cne.22170. J Comp Neurol. 2009. PMID: 19824103
-
Peripheral sensilla of some lower invertebrates: the Platyhelminthes and Nematoda.Microsc Res Tech. 1992 Aug 1;22(3):285-97. doi: 10.1002/jemt.1070220306. Microsc Res Tech. 1992. PMID: 1504355 Review.
Cited by
-
Ordered arrangement of dendrites within a C. elegans sensory nerve bundle.Elife. 2018 Aug 20;7:e35825. doi: 10.7554/eLife.35825. Elife. 2018. PMID: 30117807 Free PMC article.
-
Structure, Distribution, and Function of Neuronal/Synaptic Spinules and Related Invaginating Projections.Neuromolecular Med. 2015 Sep;17(3):211-40. doi: 10.1007/s12017-015-8358-6. Epub 2015 May 26. Neuromolecular Med. 2015. PMID: 26007200 Free PMC article. Review.
-
Resolving phylogenetic incongruence to articulate homology and phenotypic evolution: a case study from Nematoda.Proc Biol Sci. 2010 May 7;277(1686):1299-307. doi: 10.1098/rspb.2009.2195. Epub 2010 Jan 27. Proc Biol Sci. 2010. PMID: 20106846 Free PMC article. Review.
-
Evolution of lateralized gustation in nematodes.bioRxiv [Preprint]. 2025 Mar 6:2024.08.31.610597. doi: 10.1101/2024.08.31.610597. bioRxiv. 2025. Update in: Elife. 2025 Jun 30;14:RP103796. doi: 10.7554/eLife.103796. PMID: 39282255 Free PMC article. Updated. Preprint.
-
Evolution of neuronal anatomy and circuitry in two highly divergent nematode species.Elife. 2019 Sep 17;8:e47155. doi: 10.7554/eLife.47155. Elife. 2019. PMID: 31526477 Free PMC article.
References
-
- Albert PS, Riddle DL. Developmental alterations in sensory neuroanatomy of the Caenorhabditis elegans dauer larva. J Comp Neurol. 1983;219:461–481. - PubMed
-
- Ashton FT, Bhopale VM, Fine AE, Schad GA. Sensory neuroanatomy of a skin-penetrating nematode parasite: Strongyloides stercoralis. I. Amphidial neurons. J Comp Neurol. 1995;357:281–295. - PubMed
-
- Ashton FT, Bhopale VM, Holt D, Smith G, Schad GA. Developmental switching in the parasitic nematode Strongyloides stercoralis is controlled by the ASF and ASI amphidial neurons. J Parasitol. 1998;84:691–695. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

