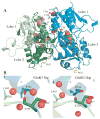
Structure of the S1S2 glutamate binding domain of GLuR3
- PMID: 19003990
- PMCID: PMC2732992
- DOI: 10.1002/prot.22274
Structure of the S1S2 glutamate binding domain of GLuR3
Abstract
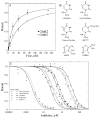
Glutamate receptors are the most prevalent excitatory neurotransmitter receptors in the vertebrate central nervous system. Determining the structural differences between the binding sites of different subtypes is crucial to our understanding of neuronal circuits and to the development of subtype specific drugs. The structures of the binding domain (S1S2) of the GluR3 (flip) AMPA receptor subunit bound to glutamate and AMPA and the GluR2 (flop) subunit bound to glutamate were determined by X-ray crystallography to 1.9, 2.1, and 1.55 A, respectively. Overall, the structure of GluR3 (flip) S1S2 is very similar to GluR2 (flop) S1S2 (backbone RMSD of 0.30 +/- 0.05 for glutamate-bound and 0.26 +/- 0.01 for AMPA-bound). The differences in the flip and flop isoforms are subtle and largely arise from one hydrogen bond across the dimer interface and associated water molecules. Comparison of the binding affinity for various agonists and partial agonists suggest that the S1S2 domains of GluR2 and GluR3 show only small differences in affinity, unlike what is found for the intact receptors (with the exception of one ligand, Cl-HIBO, which has a 10-fold difference in affinity for GluR2 vs. GluR3).
Figures


References
-
- Dingledine R, Borges K, Bowie D, Traynelis S. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. - PubMed
-
- Asztely F, Gustafsson B. Ionotropic glutamate receptors. Their possible role in the expression of hippocampal synaptic plasticity. Mol Neurobiol. 1996;12(1):1–11. - PubMed
-
- Oswald RE. Ionotropic glutamate receptor recognition and activation. Advances in Protein Chemistry. 2004;68:313–349. - PubMed
-
- Mayer ML, Olson R, Gouaux E. Mechanisms for ligand binding to GluR0 ion channels: crystal structures of the glutamate and serine complexes and a closed apo state. J Mol Biol. 2001;311(4):815–836. - PubMed
-
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28(1):165–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

