Inflammatory papillomatous hyperplasia and epidermal necrosis in a transgenic rat for HIV-1
- PMID: 19004620
- PMCID: PMC3125122
- DOI: 10.1016/j.jdermsci.2008.08.015
Inflammatory papillomatous hyperplasia and epidermal necrosis in a transgenic rat for HIV-1
Abstract
Background: Skin lesions commonly affect AIDS patients. The pathogenesis of certain dermatologic disorders primarily associated to HIV-1 is unclear, and better forms of therapy for these conditions need to be discovered. Transgenic animal models represent a novel approach for the study of these disorders and for the quest of more effective forms of treatment.
Objective: Characterize this HIV-1 transgenic rat as a model to study skin diseases related to HIV/AIDS.
Methods: A transgenic rat was developed, using an HIV-1 construct with deleted gag and pol genes. Morphological and genotypical evaluations were followed by cytokine profile characterization of the lesions.
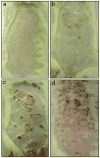
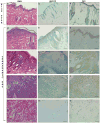
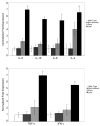
Results: We report the characterization of a colony of HIV-1 transgenic rats that developed skin lesions in a frequency of 22.5%. Cutaneous expression of functional HIV-1 transgenes correlated precisely with the severity of the phenotype. In early stages, rats manifested localized areas of xerosis and dispersed papulosquamous lesions. These hyperplastic manifestations were observed in conjunction with an increased epidermal expression of tat protein and a Th1/Th2 profile of cytokines. As the lesions progressed, they formed inflammatory plaques that subsequently ulcerated. Histologically, these lesions displayed a profound lymphocytic infiltrate, epidermal necrosis, and a marked increase of both Th1 and Th2 derived cytokines. Moreover, the presence of circulating IgG antibodies against HIV-1 gp120 was detected.
Conclusion: This animal model as other HIV-1 transgenic mice described in the past, is not able to fully explain the myriad of skin findings that can occur in HIV-infected humans; however, it represents a potential animal model system for the study of immune-mediated inflammatory skin diseases.
Figures


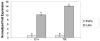
Similar articles
-
Hypoxia-inducible factor-1 α/platelet derived growth factor axis in HIV-associated pulmonary vascular remodeling.Respir Res. 2011 Aug 5;12(1):103. doi: 10.1186/1465-9921-12-103. Respir Res. 2011. PMID: 21819559 Free PMC article.
-
Cutaneous disorders and viral gene expression in HIV-1 transgenic mice.AIDS Res Hum Retroviruses. 1993 Mar;9(3):267-75. doi: 10.1089/aid.1993.9.267. AIDS Res Hum Retroviruses. 1993. PMID: 8471318
-
Vesicular Contact Reaction May Progress into Erythema Multiforme.Acta Dermatovenerol Croat. 2016 Dec;24(4):307-309. Acta Dermatovenerol Croat. 2016. PMID: 28128086 Review.
-
Impairment of T cell development and acute inflammatory response in HIV-1 Tat transgenic mice.Sci Rep. 2015 Sep 7;5:13864. doi: 10.1038/srep13864. Sci Rep. 2015. PMID: 26343909 Free PMC article.
-
Human immunodeficiency virus-1 Tat protein: immunological facets of a transcriptional activator.Indian J Biochem Biophys. 2007 Oct;44(5):269-75. Indian J Biochem Biophys. 2007. PMID: 18341200 Review.
Cited by
-
Vitamin A deficiency and behavioral and motor deficits in the human immunodeficiency virus type 1 transgenic rat.J Neurovirol. 2009 Sep;15(5-6):380-9. doi: 10.3109/13550280903350200. J Neurovirol. 2009. PMID: 19995129 Free PMC article.
-
Glial TNFα in the spinal cord regulates neuropathic pain induced by HIV gp120 application in rats.Mol Pain. 2011 May 20;7:40. doi: 10.1186/1744-8069-7-40. Mol Pain. 2011. PMID: 21599974 Free PMC article.
-
Effect of Cocaine on Pulmonary Vascular Remodeling and Hemodynamics in Human Immunodeficiency Virus-Transgenic Rats.Am J Respir Cell Mol Biol. 2016 Aug;55(2):201-12. doi: 10.1165/rcmb.2015-0264OC. Am J Respir Cell Mol Biol. 2016. PMID: 26820592 Free PMC article.
-
New insights into HIV-1-primary skin disorders.J Int AIDS Soc. 2011 Jan 24;14:5. doi: 10.1186/1758-2652-14-5. J Int AIDS Soc. 2011. PMID: 21261982 Free PMC article. Review.
-
Quantitation of parvalbumin+ neurons and human immunodeficiency virus type 1 (HIV-1) regulatory gene expression in the HIV-1 transgenic rat: effects of vitamin A deficiency and morphine.J Neurovirol. 2010 Feb;16(1):33-40. doi: 10.3109/13550280903555712. J Neurovirol. 2010. PMID: 20113193 Free PMC article.
References
-
- Zalla MJ, Su WP, Fransway AF. Dermatologic manifestations of human immunodeficiency virus infection. Mayo Clin Proc. 1992;67:1089–108. - PubMed
-
- Calista D, Morri M, Stagno A, Boschini A. Changing morbidity of cutaneous diseases in patients with HIV after the introduction of highly active anti-retroviral therapy including a protease inhibitor. Am J Clin Dermatol. 2002;3:59–62. - PubMed
-
- Bernstein WB, Little RF, Wilson WH, Yarchoan R. Acquired immunodeficiency syndrome-related malignancies in the era of highly active antiretroviral therapy. Int J Hematol. 2006;84:3–11. - PubMed
-
- Maurer TA. Dermatologic manifestations of HIV infection. Top HIV Med. 2005;13:149–54. - PubMed
-
- Uthayakumar S, Nandwani R, Drinkwater T, Nayagam AT, Darley CR. The prevalence of skin disease in HIV infection and its relationship to the degree of immunosuppression. Br J Dermatol. 1997;137:595–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

