High tolerance for ionizable residues in the hydrophobic interior of proteins
- PMID: 19004768
- PMCID: PMC2584708
- DOI: 10.1073/pnas.0805113105
High tolerance for ionizable residues in the hydrophobic interior of proteins
Abstract
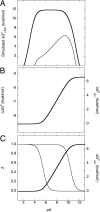
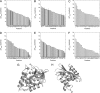
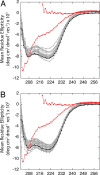
Internal ionizable groups are quite rare in water-soluble globular proteins. Presumably, this reflects the incompatibility between charges and the hydrophobic environment in the protein interior. Here we show that proteins can have an inherently high tolerance for internal ionizable groups. The 25 internal positions in staphylococcal nuclease were substituted one at a time with Lys, Glu, or Asp without abolishing enzymatic activity and without detectable changes in the conformation of the protein. Similar results with substitutions of 6 randomly chosen internal positions in ribonuclease H with Lys and Glu suggest that the ability of proteins to tolerate internal ionizable groups might be a property common to many proteins. Eighty-six of the 87 substitutions made were destabilizing, but in all but one case the proteins remained in the native state at neutral pH. By comparing the stability of each variant protein at two different pH values it was established that the pK(a) values of most of the internal ionizable groups are shifted; many of the internal ionizable groups are probably neutral at physiological pH values. These studies demonstrate that special structural adaptations are not needed for ionizable groups to exist stably in the hydrophobic interior of proteins. The studies suggest that enzymes and other proteins that use internal ionizable groups for functional purposes could have evolved through the random accumulation of mutations that introduced ionizable groups at internal positions, followed by evolutionary adaptation and optimization to modulate stability, dynamics, and other factors necessary for function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
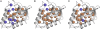
References
-
- Kauzmann W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. - PubMed
-
- Perutz MF, Kendrew JC, Watson HC. Structure and function of haemoglobin: II. Some relations betwen polypeptide chain configuration and amio acid sequence. J Mol Biol. 1965;13:669–678.
-
- Kendrew JC, et al. A partial determination by x-ray methods and its correlation with chemical data. Nature. 1961;190:666–670. - PubMed
-
- Balaji S, Aruna S, Srinivasan N. Tolerance of the substitution of buried apolar residues by charged residues in the homologous protein structures. Proteins Struct Funct Genet. 2003;53:783–791. - PubMed
-
- Kajander T, et al. Buried charged surface in proteins. Struct Fold Des. 2000;8:1203–1214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

