Deletion of the chloride transporter Slc26a9 causes loss of tubulovesicles in parietal cells and impairs acid secretion in the stomach
- PMID: 19004773
- PMCID: PMC2582584
- DOI: 10.1073/pnas.0800616105
Deletion of the chloride transporter Slc26a9 causes loss of tubulovesicles in parietal cells and impairs acid secretion in the stomach
Abstract
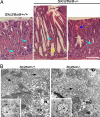
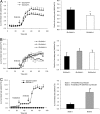
Slc26a9 is a recently identified anion transporter that is abundantly expressed in gastric epithelial cells. To study its role in stomach physiology, gene targeting was used to prepare mice lacking Slc26a9. Homozygous mutant (Slc26a9(-/-)) mice appeared healthy and displayed normal growth. Slc26a9 deletion resulted in the loss of gastric acid secretion and a moderate reduction in the number of parietal cells in mutant mice at 5 weeks of age. Immunofluorescence labeling detected the H-K-ATPase exclusively on the apical pole of gastric parietal cells in Slc26a9(-/-) mice, in contrast to the predominant cytoplasmic localization in Slc26a9(+/+) mice. Light microscopy indicated that gastric glands were dilated, and electron micrographs displayed a distinct and striking absence of tubulovesicles in parietal cells and reductions in the numbers of parietal and zymogen cells in Slc26a9(-/-) stomach. Expression studies indicated that Slc26a9 can function as a chloride conductive pathway in oocytes as well as a Cl(-)/HCO(3)(-) exchanger in cultured cells, and localization studies in parietal cells detected its presence in tubulovesicles. We propose that Slc26a9 plays an essential role in gastric acid secretion via effects on the viability of tubulovesicles/secretory canaliculi and by regulating chloride secretion in parietal cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Genetic Ablation of the ClC-2 Cl- Channel Disrupts Mouse Gastric Parietal Cell Acid Secretion.PLoS One. 2015 Sep 17;10(9):e0138174. doi: 10.1371/journal.pone.0138174. eCollection 2015. PLoS One. 2015. PMID: 26378782 Free PMC article.
-
Identification of a basolateral Cl-/HCO3- exchanger specific to gastric parietal cells.Am J Physiol Gastrointest Liver Physiol. 2003 Jun;284(6):G1093-103. doi: 10.1152/ajpgi.00454.2002. Am J Physiol Gastrointest Liver Physiol. 2003. PMID: 12736153
-
Stomachs of mice lacking the gastric H,K-ATPase alpha -subunit have achlorhydria, abnormal parietal cells, and ciliated metaplasia.J Biol Chem. 2000 Jul 14;275(28):21555-65. doi: 10.1074/jbc.M001558200. J Biol Chem. 2000. PMID: 10764766
-
The Physiology of the Gastric Parietal Cell.Physiol Rev. 2020 Apr 1;100(2):573-602. doi: 10.1152/physrev.00016.2019. Epub 2019 Oct 31. Physiol Rev. 2020. PMID: 31670611 Free PMC article. Review.
-
Functional transformation of gastric parietal cells and intracellular trafficking of ion channels/transporters in the apical canalicular membrane associated with acid secretion.Biol Pharm Bull. 2011;34(6):813-6. doi: 10.1248/bpb.34.813. Biol Pharm Bull. 2011. PMID: 21628877 Review.
Cited by
-
SLC26A9-mediated chloride secretion prevents mucus obstruction in airway inflammation.J Clin Invest. 2012 Oct;122(10):3629-34. doi: 10.1172/JCI60429. Epub 2012 Sep 4. J Clin Invest. 2012. PMID: 22945630 Free PMC article.
-
Expression of SLC26A9 in Airways and Its Potential Role in Asthma.Int J Mol Sci. 2022 Mar 10;23(6):2998. doi: 10.3390/ijms23062998. Int J Mol Sci. 2022. PMID: 35328418 Free PMC article.
-
Solution structure of the guanine nucleotide-binding STAS domain of SLC26-related SulP protein Rv1739c from Mycobacterium tuberculosis.J Biol Chem. 2011 Mar 11;286(10):8534-8544. doi: 10.1074/jbc.M110.165449. Epub 2010 Dec 29. J Biol Chem. 2011. PMID: 21190940 Free PMC article.
-
Genetic ablation of carbonic anhydrase IX disrupts gastric barrier function via claudin-18 downregulation and acid backflux.Acta Physiol (Oxf). 2018 Apr;222(4):e12923. doi: 10.1111/apha.12923. Epub 2017 Oct 19. Acta Physiol (Oxf). 2018. PMID: 28748627 Free PMC article.
-
The chloride channel/transporter Slc26a9 regulates the systemic arterial pressure and renal chloride excretion.J Mol Med (Berl). 2013 May;91(5):561-72. doi: 10.1007/s00109-012-0973-1. Epub 2012 Nov 13. J Mol Med (Berl). 2013. PMID: 23149824 Free PMC article.
References
-
- Berglindh T. Absolute dependence on chloride for acid secretion in isolated gastric glands. Gastroenterology. 1977;73:874–880. - PubMed
-
- Machen TE, McLennan WL. Na+-dependent H+ and Cl− transport in in vitro frog gastric mucosa. Am J Physiol. 1980;238:G403–G413. - PubMed
-
- Muallem S, Burnham C, Blissard D, Berglindh T, Sachs G. Electrolyte transport across the basolateral membrane of the parietal cells. J Biol Chem. 1985;260:6641–6653. - PubMed
-
- Paradiso AM, Tsien RY, Demarest JR, Machen TE. Na-H and Cl-HCO3 exchange in rabbit oxyntic cells using fluorescence microscopy. Am J Physiol. 1987;253:C30–C36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

