Horizontal gene transfer of the algal nuclear gene psbO to the photosynthetic sea slug Elysia chlorotica
- PMID: 19004808
- PMCID: PMC2584685
- DOI: 10.1073/pnas.0804968105
Horizontal gene transfer of the algal nuclear gene psbO to the photosynthetic sea slug Elysia chlorotica
Abstract
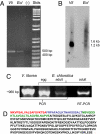
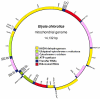
The sea slug Elysia chlorotica acquires plastids by ingestion of its algal food source Vaucheria litorea. Organelles are sequestered in the mollusc's digestive epithelium, where they photosynthesize for months in the absence of algal nucleocytoplasm. This is perplexing because plastid metabolism depends on the nuclear genome for >90% of the needed proteins. Two possible explanations for the persistence of photosynthesis in the sea slug are (i) the ability of V. litorea plastids to retain genetic autonomy and/or (ii) more likely, the mollusc provides the essential plastid proteins. Under the latter scenario, genes supporting photosynthesis have been acquired by the animal via horizontal gene transfer and the encoded proteins are retargeted to the plastid. We sequenced the plastid genome and confirmed that it lacks the full complement of genes required for photosynthesis. In support of the second scenario, we demonstrated that a nuclear gene of oxygenic photosynthesis, psbO, is expressed in the sea slug and has integrated into the germline. The source of psbO in the sea slug is V. litorea because this sequence is identical from the predator and prey genomes. Evidence that the transferred gene has integrated into sea slug nuclear DNA comes from the finding of a highly diverged psbO 3' flanking sequence in the algal and mollusc nuclear homologues and gene absence from the mitochondrial genome of E. chlorotica. We demonstrate that foreign organelle retention generates metabolic novelty ("green animals") and is explained by anastomosis of distinct branches of the tree of life driven by predation and horizontal gene transfer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Genome analysis of Elysia chlorotica Egg DNA provides no evidence for horizontal gene transfer into the germ line of this Kleptoplastic Mollusc.Mol Biol Evol. 2013 Aug;30(8):1843-52. doi: 10.1093/molbev/mst084. Epub 2013 May 2. Mol Biol Evol. 2013. PMID: 23645554 Free PMC article.
-
Molecular characterization of the Calvin cycle enzyme phosphoribulokinase in the stramenopile alga Vaucheria litorea and the plastid hosting mollusc Elysia chlorotica.Mol Plant. 2009 Nov;2(6):1384-96. doi: 10.1093/mp/ssp085. Epub 2009 Oct 30. Mol Plant. 2009. PMID: 19995736 Free PMC article.
-
LIGHT-REGULATED PHOTOSYNTHETIC GENE EXPRESSION AND PHOSPHORIBULOKINASE ENZYME ACTIVITY IN THE HETEROKONT ALGA VAUCHERIA LITOREA (XANTHOPHYCEAE) AND ITS SYMBIOTIC MOLLUSKAN PARTNER ELYSIA CHLOROTICA (GASTROPODA)(1).J Phycol. 2012 Apr;48(2):373-83. doi: 10.1111/j.1529-8817.2012.01111.x. Epub 2012 Feb 3. J Phycol. 2012. PMID: 27009727
-
Cell biology of the chloroplast symbiosis in sacoglossan sea slugs.Int Rev Cell Mol Biol. 2012;293:123-48. doi: 10.1016/B978-0-12-394304-0.00009-9. Int Rev Cell Mol Biol. 2012. PMID: 22251560 Review.
-
What was the real contribution of endosymbionts to the eukaryotic nucleus? Insights from photosynthetic eukaryotes.Cold Spring Harb Perspect Biol. 2014 Jul 1;6(7):a016014. doi: 10.1101/cshperspect.a016014. Cold Spring Harb Perspect Biol. 2014. PMID: 24984774 Free PMC article. Review.
Cited by
-
Endosymbiotic associations within protists.Philos Trans R Soc Lond B Biol Sci. 2010 Mar 12;365(1541):699-712. doi: 10.1098/rstb.2009.0188. Philos Trans R Soc Lond B Biol Sci. 2010. PMID: 20124339 Free PMC article. Review.
-
The biosynthesis of cyanobacterial sunscreen scytonemin in intertidal microbial mat communities.FEMS Microbiol Ecol. 2011 Aug;77(2):322-32. doi: 10.1111/j.1574-6941.2011.01113.x. Epub 2011 May 12. FEMS Microbiol Ecol. 2011. PMID: 21501195 Free PMC article.
-
The making of a photosynthetic animal.J Exp Biol. 2011 Jan 15;214(Pt 2):303-11. doi: 10.1242/jeb.046540. J Exp Biol. 2011. PMID: 21177950 Free PMC article.
-
Chloroplast evolution, structure and functions.F1000Prime Rep. 2014 Jun 2;6:40. doi: 10.12703/P6-40. eCollection 2014. F1000Prime Rep. 2014. PMID: 24991417 Free PMC article. Review.
-
Contribution of lateral gene transfers to the genome composition and parasitic ability of root-knot nematodes.PLoS One. 2012;7(11):e50875. doi: 10.1371/journal.pone.0050875. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226415 Free PMC article.
References
-
- Martin W, Kowallik KV. Annotated English translation of Mereschkowsky's 1905 paper Über Natur und Ursprung der Chromatophoren im Pflanzenreiche. Eur J Phycol. 1999;34:287–295.
-
- Margulis L, Sagan D. Acquiring genomes: A theory of the origins of species. New York, NY: Basic Books; 2003.
-
- Reyes-Prieto A, Weber AP, Bhattacharya D. The origin and establishment of the plastid in algae and plants. Annu Rev Genet. 2007;41:147–168. - PubMed
-
- Margulis L. Origin of Eukaryotic Cells. New Haven, CT: Yale Univ Press; 1970.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

