Biomolecular characterization of CD44-fibrin(ogen) binding: distinct molecular requirements mediate binding of standard and variant isoforms of CD44 to immobilized fibrin(ogen)
- PMID: 19004834
- PMCID: PMC2613610
- DOI: 10.1074/jbc.M805144200
Biomolecular characterization of CD44-fibrin(ogen) binding: distinct molecular requirements mediate binding of standard and variant isoforms of CD44 to immobilized fibrin(ogen)
Abstract
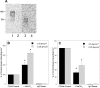
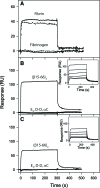
CD44 and fibrin(ogen) play critical roles in the hematogenous dissemination of tumor cells, including colon carcinomas. We recently reported that CD44 is the primary fibrin, but not fibrinogen, receptor on LS174T colon carcinomas. However, the biochemical nature of this interaction and the roles of CD44 standard (CD44s) versus CD44 variant (CD44v) isoforms in fibrin(ogen) recognition have yet to be delineated. Microspheres, coated with CD44 immunopurified from LS174T or T84 colon carcinoma cells, which express primarily CD44v, effectively bind to immobilized fibrin, but not fibrinogen, in shear flow. In contrast, CD44s from HL-60 cells binds to both immobilized fibrin and fibrinogen under flow. Use of highly specific enzymes and metabolic inhibitors reveals that LS174T CD44 binding to fibrin is dependent on O-glycosylation of CD44, whereas CD44s-fibrin(ogen) interaction has an absolute requirement for N-, but not O-, linked glycans. The presence of chondroitin and dermatan sulfate on CD44 standard and variant isoforms facilitates fibrin recognition. Use of the anti-CD44 function-blocking monoclonal antibody Hermes-1 nearly abolishes binding of LS174T CD44 to fibrin, although it has no effect on CD44s-fibrin(ogen) interaction. The CD44-binding site is localized within the N-terminal portion of the fibrin beta chains, including amino acid residues (beta15-66). Surface plasmon resonance experiments revealed high affinity binding of immobilized CD44 with solubilized fibrin but not fibrinogen. Collectively, these data suggest that immobilization of fibrinogen exposes a cryptic site that mediates binding to CD44s but not CD44v. Our findings may provide a rational basis for designing novel therapeutic strategies to combat metastasis.
Figures
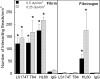
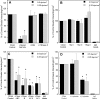

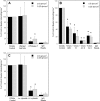

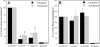
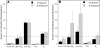
Similar articles
-
Distinct kinetic and molecular requirements govern CD44 binding to hyaluronan versus fibrin(ogen).Biophys J. 2012 Aug 8;103(3):415-423. doi: 10.1016/j.bpj.2012.06.039. Biophys J. 2012. PMID: 22947857 Free PMC article.
-
PDGF suppresses the sulfation of CD44v and potentiates CD44v-mediated binding of colon carcinoma cells to fibrin under flow.PLoS One. 2012;7(10):e41472. doi: 10.1371/journal.pone.0041472. Epub 2012 Oct 8. PLoS One. 2012. PMID: 23056168 Free PMC article.
-
The dual role of CD44 as a functional P-selectin ligand and fibrin receptor in colon carcinoma cell adhesion.Am J Physiol Cell Physiol. 2008 Apr;294(4):C907-16. doi: 10.1152/ajpcell.00463.2007. Epub 2008 Jan 30. Am J Physiol Cell Physiol. 2008. PMID: 18234849
-
CD44: structure, function, and association with the malignant process.Adv Cancer Res. 1997;71:241-319. doi: 10.1016/s0065-230x(08)60101-3. Adv Cancer Res. 1997. PMID: 9111868 Review.
-
CD44 isoform-cytoskeleton interaction in oncogenic signaling and tumor progression.Front Biosci. 1998 Jul 1;3:d637-49. doi: 10.2741/a308. Front Biosci. 1998. PMID: 9634539 Review.
Cited by
-
Effect of CD44 binding peptide conjugated to an engineered inert matrix on maintenance of breast cancer stem cells and tumorsphere formation.PLoS One. 2013;8(3):e59147. doi: 10.1371/journal.pone.0059147. Epub 2013 Mar 18. PLoS One. 2013. PMID: 23527117 Free PMC article.
-
FGA influences invasion and metastasis of hepatocellular carcinoma through the PI3K/AKT pathway.Aging (Albany NY). 2024 Jul 9;16(19):12806-12819. doi: 10.18632/aging.206011. Epub 2024 Jul 9. Aging (Albany NY). 2024. PMID: 39227068 Free PMC article.
-
Immunological Roles of Elevated Plasma Levels of Matricellular Proteins in Japanese Patients with Pulmonary Tuberculosis.Int J Mol Sci. 2016 Dec 22;18(1):19. doi: 10.3390/ijms18010019. Int J Mol Sci. 2016. PMID: 28025511 Free PMC article.
-
Role of coagulation in the recruitment of colon adenocarcinoma cells to thrombus under shear.Am J Physiol Cell Physiol. 2013 Nov 1;305(9):C951-9. doi: 10.1152/ajpcell.00185.2013. Epub 2013 Jul 31. Am J Physiol Cell Physiol. 2013. PMID: 23903698 Free PMC article.
-
CD44-fibrinogen binding promotes bleeding in acute promyelocytic leukemia by in situ fibrin(ogen) deposition.Blood Adv. 2022 Aug 9;6(15):4617-4633. doi: 10.1182/bloodadvances.2022006980. Blood Adv. 2022. PMID: 35511736 Free PMC article.
References
-
- Ponta, H., Sherman, L., and Herrlich, P. A. (2003) Nat. Rev. Mol. Cell Biol. 4 33-45 - PubMed
-
- Martin, T. A., Harrison, G., Mansel, R. E., and Jiang, W. G. (2003) Crit. Rev. Oncol. Hematol. 46 165-186 - PubMed
-
- Harada, N., Mizoi, T., Kinouchi, M., Hoshi, K., Ishii, S., Shiiba, K., Sasaki, I., and Matsuno, S. (2001) Int. J. Cancer 91 67-75 - PubMed
-
- Hofmann, M., Rudy, W., Zoller, M., Tolg, C., Ponta, H., Herrlich, P., and Gunthert, U. (1991) Cancer Res. 51 5292-5297 - PubMed
-
- Wielenga, V. J., Heider, K. H., Offerhaus, G. J., Adolf, G. R., van den Berg, F. M., Ponta, H., Herrlich, P., and Pals, S. T. (1993) Cancer Res. 53 4754-4756 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

