Dual requirement for Pax6 in retinal progenitor cells
- PMID: 19004853
- PMCID: PMC4231183
- DOI: 10.1242/dev.028308
Dual requirement for Pax6 in retinal progenitor cells
Abstract
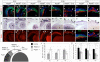
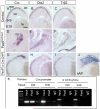
Throughout the developing central nervous system, pre-patterning of the ventricular zone into discrete neural progenitor domains is one of the predominant strategies used to produce neuronal diversity in a spatially coordinated manner. In the retina, neurogenesis proceeds in an intricate chronological and spatial sequence, yet it remains unclear whether retinal progenitor cells (RPCs) display intrinsic heterogeneity at any given time point. Here, we performed a detailed study of RPC fate upon temporally and spatially confined inactivation of Pax6. Timed genetic removal of Pax6 appeared to unmask a cryptic divergence of RPCs into qualitatively divergent progenitor pools. In the more peripheral RPCs under normal circumstances, Pax6 seemed to prevent premature activation of a photoreceptor-differentiation pathway by suppressing expression of the transcription factor Crx. More centrally, Pax6 contributed to the execution of the comprehensive potential of RPCs: Pax6 ablation resulted in the exclusive generation of amacrine interneurons. Together, these data suggest an intricate dual role for Pax6 in retinal neurogenesis, while pointing to the cryptic divergence of RPCs into distinct progenitor pools.
Figures




References
-
- Alexiades MR, Cepko CL. Subsets of retinal progenitors display temporally regulated and distinct biases in the fates of their progeny. Development. 1997;124:1119–1131. - PubMed
-
- Applebury ML, Farhangfar F, Glosmann M, Hashimoto K, Kage K, Robbins JT, Shibusawa N, Wondisford FE, Zhang H. Transient expression of thyroid hormone nuclear receptor TRbeta2 sets S opsin patterning during cone photoreceptor genesis. Dev. Dyn. 2007;236:1203–1212. - PubMed
-
- Aramant R, Seiler M, Ehinger B, Bergstrom A, Adolph AR, Turner JE. Neuronal markers in rat retinal grafts. Brain Res. Dev. Brain Res. 1990;53:47–61. - PubMed
-
- Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306:869–871. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

