Canonical Wnt signaling is required for the maintenance of dorsal retinal identity
- PMID: 19004855
- PMCID: PMC2667153
- DOI: 10.1242/dev.027367
Canonical Wnt signaling is required for the maintenance of dorsal retinal identity
Abstract
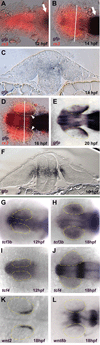
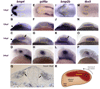
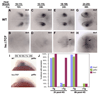
Accurate retinotectal axon pathfinding depends upon the correct establishment of dorsal-ventral retinal polarity. We show that dorsal retinal gene expression is regulated by Wnt signaling in the dorsal retinal pigment epithelium (RPE). We find that a Wnt reporter transgene and Wnt pathway components are expressed in the dorsal RPE beginning at 14-16 hours post-fertilization. In the absence of Wnt signaling, tbx5 and Bmp genes initiate normal dorsal retinal expression but are not maintained. The expression of these genes is rescued by the downstream activation of Wnt signaling, and tbx5 is rescued by Bmp signaling. Furthermore, activation of Wnt signaling cannot rescue tbx5 in the absence of Bmp signaling, suggesting that Wnt signaling maintains dorsal retinal gene expression by regulating Bmp signaling. We present a model in which dorsal RPE-derived Wnt activity maintains the expression of Bmp ligands in the dorsal retina, thus coordinating the patterning of these two ocular tissues.
Figures



Similar articles
-
Sfrp1a and Sfrp5 function as positive regulators of Wnt and BMP signaling during early retinal development.Dev Biol. 2014 Apr 15;388(2):192-204. doi: 10.1016/j.ydbio.2014.01.012. Epub 2014 Jan 20. Dev Biol. 2014. PMID: 24457098
-
Wnt-regulated dynamics of positional information in zebrafish somitogenesis.Development. 2014 Mar;141(6):1381-91. doi: 10.1242/dev.093435. Development. 2014. PMID: 24595291 Free PMC article.
-
Gdf6a is required for the initiation of dorsal-ventral retinal patterning and lens development.Dev Biol. 2009 Sep 1;333(1):37-47. doi: 10.1016/j.ydbio.2009.06.018. Epub 2009 Jun 21. Dev Biol. 2009. PMID: 19545559
-
Heads or tails? Amphioxus and the evolution of anterior-posterior patterning in deuterostomes.Dev Biol. 2002 Jan 15;241(2):209-28. doi: 10.1006/dbio.2001.0503. Dev Biol. 2002. PMID: 11784106 Review.
-
Bone morphogenetic proteins in the early development of zebrafish.FEBS J. 2007 Jun;274(12):2960-7. doi: 10.1111/j.1742-4658.2007.05838.x. Epub 2007 May 22. FEBS J. 2007. PMID: 17521339 Review.
Cited by
-
Canonical Wnt/β-catenin signalling is essential for optic cup formation.PLoS One. 2013 Dec 4;8(12):e81158. doi: 10.1371/journal.pone.0081158. eCollection 2013. PLoS One. 2013. PMID: 24324671 Free PMC article.
-
Repurposing development genes for axonal regeneration following injury: Examining the roles of Wnt signaling.Front Cell Dev Biol. 2024 May 31;12:1417928. doi: 10.3389/fcell.2024.1417928. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38882059 Free PMC article. Review.
-
Meis1 specifies positional information in the retina and tectum to organize the zebrafish visual system.Neural Dev. 2010 Sep 1;5:22. doi: 10.1186/1749-8104-5-22. Neural Dev. 2010. PMID: 20809932 Free PMC article.
-
Cell cycle-related kinase regulates mammalian eye development through positive and negative regulation of the Hedgehog pathway.Dev Biol. 2018 Feb 1;434(1):24-35. doi: 10.1016/j.ydbio.2017.10.022. Epub 2017 Nov 21. Dev Biol. 2018. PMID: 29166577 Free PMC article.
-
BMP Signaling Interferes with Optic Chiasm Formation and Retinal Ganglion Cell Pathfinding in Zebrafish.Int J Mol Sci. 2021 Apr 27;22(9):4560. doi: 10.3390/ijms22094560. Int J Mol Sci. 2021. PMID: 33925390 Free PMC article.
References
-
- Arce L, Yokoyama NN, Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25:7492–7504. - PubMed
-
- Barbieri AM, Broccoli V, Bovolenta P, Alfano G, Marchitiello A, Mocchetti C, Crippa L, Bulfone A, Marigo V, Ballabio A, et al. Vax2 inactivation in mouse determines alteration of the eye dorsal-ventral axis, misrouting of the optic fibres and eye coloboma. Development. 2002;129:805–813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

