The human papillomavirus type 8 E2 tethering protein targets the ribosomal DNA loci of host mitotic chromosomes
- PMID: 19004936
- PMCID: PMC2612381
- DOI: 10.1128/JVI.01936-08
The human papillomavirus type 8 E2 tethering protein targets the ribosomal DNA loci of host mitotic chromosomes
Abstract
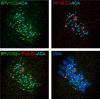
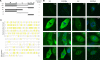
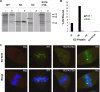
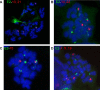
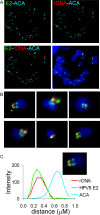
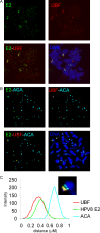
For many papillomaviruses, the viral protein E2 tethers the viral genome to the host mitotic chromosomes to ensure persistent, long-term maintenance of the genome during cell division. Our previous studies of E2 proteins from different genera of papillomaviruses have shown that they bind to different regions of the host chromosomes during mitosis. For example, bovine papillomavirus type 1 (BPV-1) E2 binds to all chromosomes as small speckles in complex with the cellular protein Brd4. In contrast, the human papillomavirus type 8 (HPV-8) E2 protein binds as large speckles at the pericentromeric regions of chromosomes. Here we show that these speckles do not contain Brd4, and unlike that of BPV-1, the N-terminal Brd4-interacting domain of HPV-8 E2 is not required for chromosome binding. In contrast to BPV-1 E2, the HPV-8 E2 protein targets the short arms of acrocentric mitotic chromosomes. Furthermore, the E2 protein interacts with the repeated ribosomal DNA genes found in this location and colocalizes with UBF, the RNA polymerase I transcription factor. Therefore, HPV-8 E2 genome tethering occurs by a Brd4-independent mechanism through a novel interaction with specific regions of mitotic chromosomes. Thus, a wide range of viruses have adopted the strategy of linking their genomes to host chromosomes, but individual viruses use different chromosomal targets. Characterization of these targets will enable the development of antiviral therapies to eliminate the viral genomes from infected cells.
Figures



Similar articles
-
Interaction of the betapapillomavirus E2 tethering protein with mitotic chromosomes.J Virol. 2010 Jan;84(1):543-57. doi: 10.1128/JVI.01908-09. J Virol. 2010. PMID: 19846509 Free PMC article.
-
Phosphorylation of HPV-16 E2 at serine 243 enables binding to Brd4 and mitotic chromosomes.PLoS One. 2014 Oct 23;9(10):e110882. doi: 10.1371/journal.pone.0110882. eCollection 2014. PLoS One. 2014. PMID: 25340539 Free PMC article.
-
Interaction of the bovine papillomavirus E2 protein with Brd4 tethers the viral DNA to host mitotic chromosomes.Cell. 2004 Apr 30;117(3):349-60. doi: 10.1016/s0092-8674(04)00402-7. Cell. 2004. PMID: 15109495
-
Involvement of Brd4 in different steps of the papillomavirus life cycle.Virus Res. 2017 Mar 2;231:76-82. doi: 10.1016/j.virusres.2016.12.006. Epub 2016 Dec 10. Virus Res. 2017. PMID: 27965149 Free PMC article. Review.
-
The functions of papillomavirus E2 proteins.Virology. 2025 Feb;603:110387. doi: 10.1016/j.virol.2024.110387. Epub 2024 Dec 31. Virology. 2025. PMID: 39826199 Review.
Cited by
-
The JAK-STAT transcriptional regulator, STAT-5, activates the ATM DNA damage pathway to induce HPV 31 genome amplification upon epithelial differentiation.PLoS Pathog. 2013;9(4):e1003295. doi: 10.1371/journal.ppat.1003295. Epub 2013 Apr 4. PLoS Pathog. 2013. PMID: 23593005 Free PMC article.
-
A new role for human papillomavirus 16 E2: Mitotic activation of the DNA damage response to promote viral genome segregation.Tumour Virus Res. 2024 Dec;18:200291. doi: 10.1016/j.tvr.2024.200291. Epub 2024 Sep 7. Tumour Virus Res. 2024. PMID: 39245413 Free PMC article. Review.
-
Regulation of the life cycle of HPVs by differentiation and the DNA damage response.Future Microbiol. 2013 Dec;8(12):1547-57. doi: 10.2217/fmb.13.127. Future Microbiol. 2013. PMID: 24266355 Free PMC article. Review.
-
The selfish yeast plasmid utilizes the condensin complex and condensed chromatin for faithful partitioning.PLoS Genet. 2021 Jul 16;17(7):e1009660. doi: 10.1371/journal.pgen.1009660. eCollection 2021 Jul. PLoS Genet. 2021. PMID: 34270553 Free PMC article.
-
A central region in the minor capsid protein of papillomaviruses facilitates viral genome tethering and membrane penetration for mitotic nuclear entry.PLoS Pathog. 2017 May 2;13(5):e1006308. doi: 10.1371/journal.ppat.1006308. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28464022 Free PMC article.
References
-
- Banerjee, R., M. K. Weidman, S. Navarro, L. Comai, and A. Dasgupta. 2005. Modifications of both selectivity factor and upstream binding factor contribute to poliovirus-mediated inhibition of RNA polymerase I transcription. J. Gen. Virol. 862315-2322. - PubMed
-
- Bastien, N., and A. A. McBride. 2000. Interaction of the papillomavirus E2 with mitotic chromosomes. Virology 270124-134. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

