Coadministration of cidofovir and smallpox vaccine reduced vaccination side effects but interfered with vaccine-elicited immune responses and immunity to monkeypox
- PMID: 19004937
- PMCID: PMC2612404
- DOI: 10.1128/JVI.00984-08
Coadministration of cidofovir and smallpox vaccine reduced vaccination side effects but interfered with vaccine-elicited immune responses and immunity to monkeypox
Abstract
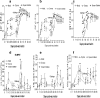
While the smallpox vaccine, Dryvax or Dryvax-derived ACAM2000, holds potential for public immunization against the spread of smallpox by bioterror, there is serious concern about Dryvax-mediated side effects. Here, we report that a single-dose vaccination regimen comprised of Dryvax and an antiviral agent, cidofovir, could reduce vaccinia viral loads after vaccination and significantly control Dryvax vaccination side effects. However, coadministration of cidofovir and Dryvax also reduced vaccine-elicited immune responses of antibody and T effector cells despite the fact that the reduced priming could be boosted as a recall response after monkeypox virus challenge. Evaluations of four different aspects of vaccine efficacy showed that coadministration of cidofovir and Dryvax compromised the Dryvax-induced immunity against monkeypox, although the covaccinated monkeys exhibited measurable protection against monkeypox compared to that of naïve controls. Thus, the single-dose coadministration of cidofovir and Dryvax effectively controlled vaccination side effects but significantly compromised vaccine-elicited immune responses and vaccine-induced immunity to monkeypox.
Figures


Similar articles
-
Co-administration of the broad-spectrum antiviral, brincidofovir (CMX001), with smallpox vaccine does not compromise vaccine protection in mice challenged with ectromelia virus.Antiviral Res. 2014 Nov;111:42-52. doi: 10.1016/j.antiviral.2014.08.003. Epub 2014 Aug 13. Antiviral Res. 2014. PMID: 25128688 Free PMC article.
-
Expansion, reexpansion, and recall-like expansion of Vgamma2Vdelta2 T cells in smallpox vaccination and monkeypox virus infection.J Virol. 2009 Nov;83(22):11959-65. doi: 10.1128/JVI.00689-09. Epub 2009 Sep 9. J Virol. 2009. PMID: 19740988 Free PMC article.
-
Clonal vaccinia virus grown in cell culture fully protects monkeys from lethal monkeypox challenge.Vaccine. 2008 Jan 24;26(4):581-8. doi: 10.1016/j.vaccine.2007.10.063. Epub 2007 Nov 20. Vaccine. 2008. PMID: 18077063 Free PMC article.
-
Treatment and Vaccination for Smallpox and Monkeypox.Adv Exp Med Biol. 2024;1451:301-316. doi: 10.1007/978-3-031-57165-7_19. Adv Exp Med Biol. 2024. PMID: 38801586 Review.
-
Efficacy of CMX001 as a prophylactic and presymptomatic antiviral agent in New Zealand white rabbits infected with rabbitpox virus, a model for orthopoxvirus infections of humans.Viruses. 2011 Feb;3(2):63-82. doi: 10.3390/v3020063. Viruses. 2011. PMID: 21369346 Free PMC article. Review.
Cited by
-
Potential use of cidofovir, brincidofovir, and tecovirimat drugs in fighting monkeypox infection: recent trends and advancements.Naunyn Schmiedebergs Arch Pharmacol. 2024 Apr;397(4):2055-2065. doi: 10.1007/s00210-023-02769-y. Epub 2023 Oct 14. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37837475 Review.
-
Mpox treatment evolution: past milestones, present advances, and future directions.Naunyn Schmiedebergs Arch Pharmacol. 2025 Feb;398(2):1057-1080. doi: 10.1007/s00210-024-03385-0. Epub 2024 Sep 3. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39225831 Review.
-
Comprehensive literature review of monkeypox.Emerg Microbes Infect. 2022 Dec;11(1):2600-2631. doi: 10.1080/22221751.2022.2132882. Emerg Microbes Infect. 2022. PMID: 36263798 Free PMC article. Review.
-
A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012.Future Virol. 2013 Feb 1;8(2):129-157. doi: 10.2217/fvl.12.130. Future Virol. 2013. PMID: 23626656 Free PMC article.
-
A review of Mpox: Biological characteristics, epidemiology, clinical features, diagnosis, treatment, and prevention strategies.Exploration (Beijing). 2024 Oct 8;5(2):20230112. doi: 10.1002/EXP.20230112. eCollection 2025 Apr. Exploration (Beijing). 2024. PMID: 40395760 Free PMC article. Review.
References
-
- Casey, C. G., J. K. Iskander, M. H. Roper, E. E. Mast, X. J. Wen, T. J. Torok, L. E. Chapman, D. L. Swerdlow, J. Morgan, J. D. Heffelfinger, C. Vitek, S. E. Reef, L. M. Hasbrouck, I. Damon, L. Neff, C. Vellozzi, M. McCauley, R. A. Strikas, and G. Mootrey. 2005. Adverse events associated with smallpox vaccination in the United States, January-October 2003. JAMA 2942734-2743. - PubMed
-
- Centers for Disease Control and Prevention. 2004. Update: adverse events following civilian smallpox vaccination—United States, 2003. MMWR Morb. Mortal. Wkly. Rep. 53106-107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

