Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells
- PMID: 19004938
- PMCID: PMC2655569
- DOI: 10.1128/JVI.01792-08
Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells
Abstract
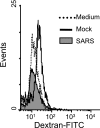
Severe acute respiratory syndrome (SARS), which is caused by a novel coronavirus (CoV), is a highly communicable disease with the lungs as the major pathological target. Although SARS likely stems from overexuberant host inflammatory responses, the exact mechanism leading to the detrimental outcome in patients remains unknown. Pulmonary macrophages (Mphi), airway epithelium, and dendritic cells (DC) are key cellular elements of the host innate defenses against respiratory infections. While pulmonary Mphi are situated at the luminal epithelial surface, DC reside abundantly underneath the epithelium. Such strategic locations of these cells within the airways make it relevant to investigate their likely impact on SARS pathogenesis subsequent to their interaction with infected lung epithelial cells. To study this, we established highly polarized human lung epithelial Calu-3 cells by using the Transwell culture system. Here we report that supernatants harvested from the apical and basolateral domains of infected Calu-3 cells are potent in modulating the intrinsic functions of Mphi and DC, respectively. They prompted the production of cytokines by both Mphi and DC and selectively induced CD40 and CD86 expression only on DC. However, they compromised the abilities of the DC and Mphi in priming naïve T cells and phagocytosis, respectively. We also identified interleukin-6 (IL-6) and IL-8 as key SARS-CoV-induced epithelial cytokines capable of inhibiting the T-cell-priming ability of DC. Taken together, our results provide insights into the molecular and cellular bases of the host antiviral innate immunity within the lungs that eventually lead to an exacerbated inflammatory cascades and severe tissue damage in SARS patients.
Figures

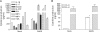
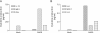
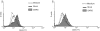

Similar articles
-
Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection.J Immunol. 2005 Jun 15;174(12):7977-85. doi: 10.4049/jimmunol.174.12.7977. J Immunol. 2005. PMID: 15944304
-
SARS-CoV-2 Isolates Show Impaired Replication in Human Immune Cells but Differential Ability to Replicate and Induce Innate Immunity in Lung Epithelial Cells.Microbiol Spectr. 2021 Sep 3;9(1):e0077421. doi: 10.1128/Spectrum.00774-21. Epub 2021 Aug 11. Microbiol Spectr. 2021. PMID: 34378952 Free PMC article.
-
SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium.Virus Res. 2008 Apr;133(1):33-44. doi: 10.1016/j.virusres.2007.03.013. Epub 2007 Apr 23. Virus Res. 2008. PMID: 17451829 Free PMC article. Review.
-
Modeling the early events of severe acute respiratory syndrome coronavirus infection in vitro.J Virol. 2006 Mar;80(6):2684-93. doi: 10.1128/JVI.80.6.2684-2693.2006. J Virol. 2006. PMID: 16501078 Free PMC article.
-
Pathogenesis of severe acute respiratory syndrome.Curr Opin Immunol. 2005 Aug;17(4):404-10. doi: 10.1016/j.coi.2005.05.009. Curr Opin Immunol. 2005. PMID: 15950449 Free PMC article. Review.
Cited by
-
Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications.Ther Adv Respir Dis. 2020 Jan-Dec;14:1753466620933508. doi: 10.1177/1753466620933508. Ther Adv Respir Dis. 2020. PMID: 32539627 Free PMC article. Review.
-
Alpha-defensins: risk factor for thrombosis in COVID-19 infection.Br J Haematol. 2021 Jul;194(1):44-52. doi: 10.1111/bjh.17503. Epub 2021 May 30. Br J Haematol. 2021. PMID: 34053084 Free PMC article. Clinical Trial.
-
Severe Acute Respiratory Syndrome Coronavirus-2 - A Surge of CoronaVirus Disease-2019: An Epidemiological Study in Coimbatore District.J Pharm Bioallied Sci. 2022 Jul;14(Suppl 1):S360-S363. doi: 10.4103/jpbs.jpbs_124_22. Epub 2022 Jul 13. J Pharm Bioallied Sci. 2022. PMID: 36110629 Free PMC article.
-
Multisystem inflammatory syndrome in children related to COVID-19: A New York City experience.J Med Virol. 2021 Jan;93(1):424-433. doi: 10.1002/jmv.26224. Epub 2020 Oct 5. J Med Virol. 2021. PMID: 32584487 Free PMC article.
-
Behavioral and Dietary Habits That Could Influence Both COVID-19 and Non-Communicable Civilization Disease Prevention-What Have We Learned Up to Now?Medicina (Kaunas). 2022 Nov 21;58(11):1686. doi: 10.3390/medicina58111686. Medicina (Kaunas). 2022. PMID: 36422225 Free PMC article. Review.
References
-
- Akagawa, K. S. 2002. Functional heterogeneity of colony-stimulating factor-induced human monocyte-derived macrophages. Int. J. Hematol. 7627-34. - PubMed
-
- Anderson, J. M. 2001. Molecular structure of tight junctions and their role in epithelial transport. News Physiol. Sci. 16126-130. - PubMed
-
- Ardavin, C., H. G. Martinez del, P. Martin, F. Anjuere, C. F. Arias, A. R. Marin, S. Ruiz, V. Parrillas, and H. Hernandez. 2001. Origin and differentiation of dendritic cells. Trends Immunol. 22691-700. - PubMed
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. - PubMed
-
- Cereijido, M., L. Gonzalez-Mariscal, and R. G. Contreras. 1988. Epithelial tight junctions. Am. Rev. Respir. Dis. 138S17-S21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

