Protein kinase PKR mediates the apoptosis induction and growth restriction phenotypes of C protein-deficient measles virus
- PMID: 19004947
- PMCID: PMC2612345
- DOI: 10.1128/JVI.01669-08
Protein kinase PKR mediates the apoptosis induction and growth restriction phenotypes of C protein-deficient measles virus
Abstract
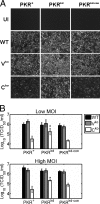
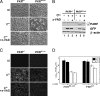
The measles virus (MV) accessory proteins V and C play important roles in MV replication and pathogenesis. Infection with recombinant MV lacking either V or C causes more cell death than infection with the parental vaccine-equivalent virus (MVvac), and C-deficient virus grows poorly relative to the parental virus. Here, we show that a major effector of the C phenotype is the RNA-dependent protein kinase PKR. Using human HeLa cells stably deficient in PKR as a result of RNA interference-mediated knockdown (PKR(kd) cells), we demonstrated that a reduction in PKR partially rescued the growth defect of C knockout (C(ko)) virus but had no effect on the growth of either wild-type (WT) or V knockout (V(ko)) virus. Increased growth of the C(ko) virus in PKR(kd) cells correlated with increased viral protein expression, while defective growth and decreased protein expression in PKR-sufficient cells correlated with increased phosphorylation of PKR and the alpha subunit of eukaryotic initiation factor 2. Furthermore, infection with WT, V(ko), or especially C(ko) virus caused significantly less apoptosis in PKR(kd) cells than in PKR-sufficient cells. Although apoptosis induced by C(ko) virus infection in PKR-sufficient cells was blocked by a caspase antagonist, the growth of C(ko) virus was not restored to the WT level by treatment with this pharmacologic inhibitor. Taken together, these results indicate that PKR plays an important antiviral role during MV infection but that the virus growth restriction by PKR is not dependent upon the induction of apoptosis. Furthermore, the results establish that a principal function of the MV C protein is to antagonize the proapoptotic and antiviral activities of PKR.
Figures



Similar articles
-
RNA-specific adenosine deaminase ADAR1 suppresses measles virus-induced apoptosis and activation of protein kinase PKR.J Biol Chem. 2009 Oct 23;284(43):29350-6. doi: 10.1074/jbc.M109.045146. Epub 2009 Aug 25. J Biol Chem. 2009. PMID: 19710021 Free PMC article.
-
Loss of protein kinase PKR expression in human HeLa cells complements the vaccinia virus E3L deletion mutant phenotype by restoration of viral protein synthesis.J Virol. 2008 Jan;82(2):840-8. doi: 10.1128/JVI.01891-07. Epub 2007 Oct 24. J Virol. 2008. PMID: 17959656 Free PMC article.
-
Protein kinase PKR catalytic activity is required for the PKR-dependent activation of mitogen-activated protein kinases and amplification of interferon beta induction following virus infection.Virology. 2012 Jun 5;427(2):208-16. doi: 10.1016/j.virol.2012.01.029. Epub 2012 Mar 3. Virology. 2012. PMID: 22381929 Free PMC article.
-
Tipping the balance: antagonism of PKR kinase and ADAR1 deaminase functions by virus gene products.J Interferon Cytokine Res. 2009 Sep;29(9):477-87. doi: 10.1089/jir.2009.0065. J Interferon Cytokine Res. 2009. PMID: 19715457 Free PMC article. Review.
-
Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): mechanism of action.Apoptosis. 2000 Apr;5(2):107-14. doi: 10.1023/a:1009664109241. Apoptosis. 2000. PMID: 11232238 Review.
Cited by
-
Extensive editing of cellular and viral double-stranded RNA structures accounts for innate immunity suppression and the proviral activity of ADAR1p150.PLoS Biol. 2018 Nov 29;16(11):e2006577. doi: 10.1371/journal.pbio.2006577. eCollection 2018 Nov. PLoS Biol. 2018. PMID: 30496178 Free PMC article.
-
Adenosine deaminase acting on RNA (ADAR1), a suppressor of double-stranded RNA-triggered innate immune responses.J Biol Chem. 2019 Feb 1;294(5):1710-1720. doi: 10.1074/jbc.TM118.004166. J Biol Chem. 2019. PMID: 30710018 Free PMC article. Review.
-
The PKR activator, PACT, becomes a PKR inhibitor during HIV-1 replication.Retrovirology. 2013 Sep 11;10:96. doi: 10.1186/1742-4690-10-96. Retrovirology. 2013. PMID: 24020926 Free PMC article.
-
C Protein is Essential for Canine Distemper Virus Virulence and Pathogenicity in Ferrets.J Virol. 2021 Feb 15;95(4):e01840-20. doi: 10.1128/JVI.01840-20. Epub 2020 Nov 25. J Virol. 2021. PMID: 33239455 Free PMC article.
-
Host WD repeat-containing protein 5 inhibits protein kinase R-mediated integrated stress response during measles virus infection.J Virol. 2024 Sep 17;98(9):e0102024. doi: 10.1128/jvi.01020-24. Epub 2024 Aug 28. J Virol. 2024. PMID: 39194235 Free PMC article.
References
-
- Bankamp, B., J. Wilson, W. J. Bellini, and P. A. Rota. 2005. Identification of naturally occurring amino acid variations that affect the ability of the measles virus C protein to regulate genome replication and transcription. Virology 336120-129. - PubMed
-
- Bolt, G., and I. R. Pedersen. 1998. The role of subtilisin-like proprotein convertases for cleavage of the measles virus fusion glycoprotein in different cell types. Virology 252387-398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

