Heterogenic feedback between hindlimb extensors in the spontaneously locomoting premammillary cat
- PMID: 19005003
- PMCID: PMC2637003
- DOI: 10.1152/jn.90338.2008
Heterogenic feedback between hindlimb extensors in the spontaneously locomoting premammillary cat
Abstract
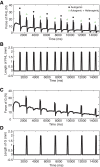
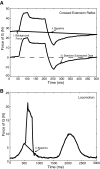
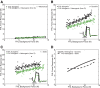
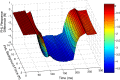
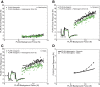
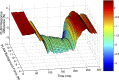
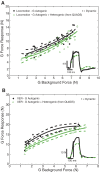
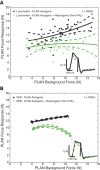
Electrophysiological studies in anesthetized animals have revealed that pathways carrying force information from Golgi tendon organs in antigravity muscles mediate widespread inhibition among other antigravity muscles in the feline hindlimb. More recent evidence in paralyzed or nonparalyzed decerebrate cats has shown that some inhibitory pathways are suppressed and separate excitatory pathways from Golgi tendon organ afferents are opened on the transition from steady force production to locomotor activity. To obtain additional insight into the functions of these pathways during locomotion, we investigated the distribution of force-dependent inhibition and excitation during spontaneous locomotion and during constant force exertion in the premammillary decerebrate cat. We used four servo-controlled stretching devices to apply controlled stretches in various combinations to the gastrocnemius muscles (G), plantaris muscle (PLAN), flexor hallucis longus muscle (FHL), and quadriceps muscles (QUADS) during treadmill stepping and the crossed-extension reflex (XER). We recorded the force responses from the same muscles and were therefore able to evaluate autogenic (intramuscular) and heterogenic (intermuscular) reflexes among this set of muscles. In previous studies using the intercollicular decerebrate cat, heterogenic inhibition among QUADS, G, FHL, and PLAN was bidirectional. During treadmill stepping, heterogenic feedback from QUADS onto G and G onto PLAN and FHL remained inhibitory and was force-dependent. However, heterogenic inhibition from PLAN and FHL onto G, and from G onto QUADS, was weaker than during the XER. We propose that pathways mediating heterogenic inhibition may remain inhibitory under some forms of locomotion on a level surface but that the strengths of these pathways change to result in a proximal to distal gradient of inhibition. The potential contributions of heterogenic inhibition to interjoint coordination and limb stability are discussed.
Figures



Similar articles
-
Mechanical actions of heterogenic reflexes linking long toe flexors with ankle and knee extensors of the cat hindlimb.J Neurophysiol. 1994 Mar;71(3):1096-110. doi: 10.1152/jn.1994.71.3.1096. J Neurophysiol. 1994. PMID: 8201405
-
Mechanical actions of heterogenic reflexes among ankle stabilizers and their interactions with plantarflexors of the cat hindlimb.J Neurophysiol. 1996 May;75(5):2050-70. doi: 10.1152/jn.1996.75.5.2050. J Neurophysiol. 1996. PMID: 8734603
-
Receptor mechanisms underlying heterogenic reflexes among the triceps surae muscles of the cat.J Neurophysiol. 1999 Feb;81(2):467-78. doi: 10.1152/jn.1999.81.2.467. J Neurophysiol. 1999. PMID: 10036251
-
Control of locomotion in the decerebrate cat.Prog Neurobiol. 1996 Aug;49(5):481-515. doi: 10.1016/0301-0082(96)00028-7. Prog Neurobiol. 1996. PMID: 8895997 Review.
-
The implications of force feedback for the lambda model.Adv Exp Med Biol. 2009;629:663-79. doi: 10.1007/978-0-387-77064-2_36. Adv Exp Med Biol. 2009. PMID: 19227527 Review.
Cited by
-
Unpredictable elbow joint perturbation during reaching results in multijoint motor equivalence.J Neurophysiol. 2011 Sep;106(3):1424-36. doi: 10.1152/jn.00163.2011. Epub 2011 Jun 15. J Neurophysiol. 2011. PMID: 21676927 Free PMC article.
-
Distributed force feedback in the spinal cord and the regulation of limb mechanics.J Neurophysiol. 2018 Mar 1;119(3):1186-1200. doi: 10.1152/jn.00216.2017. Epub 2017 Dec 6. J Neurophysiol. 2018. PMID: 29212914 Free PMC article. Review.
-
ROLE OF FORELIMB MORPHOLOGY IN MUSCLE SENSORIMOTOR FUNCTIONS DURING LOCOMOTION IN THE CAT.bioRxiv [Preprint]. 2024 Jul 16:2024.07.11.603106. doi: 10.1101/2024.07.11.603106. bioRxiv. 2024. Update in: J Physiol. 2025 Jan;603(2):447-487. doi: 10.1113/JP287448. PMID: 39071389 Free PMC article. Updated. Preprint.
-
Tuning of feedforward control enables stable muscle force-length dynamics after loss of autogenic proprioceptive feedback.Elife. 2020 Jun 23;9:e53908. doi: 10.7554/eLife.53908. Elife. 2020. PMID: 32573432 Free PMC article.
-
Musculotendon adaptations and preservation of spinal reflex pathways following agonist-to-antagonist tendon transfer.Physiol Rep. 2017 May;5(9):e13201. doi: 10.14814/phy2.13201. Physiol Rep. 2017. PMID: 28468849 Free PMC article.
References
-
- Bonasera SJ Toward a Neural Representation of the Feline Ankle Joint. Atlanta, GA: Emory University, 1994.
-
- Bonasera SJ, Nichols TR. Mechanical actions of heterogenic reflexes linking long toe flexors and extensors of the knee and ankle in the cat. J Neurophysiol 71: 1096–1110, 1994. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

