HNF-1beta regulates transcription of the PKD modifier gene Kif12
- PMID: 19005009
- PMCID: PMC2615735
- DOI: 10.1681/ASN.2008020238
HNF-1beta regulates transcription of the PKD modifier gene Kif12
Abstract
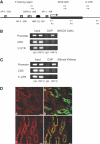
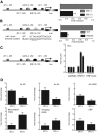
Hepatocyte nuclear factor-1beta (HNF-1beta) is a transcription factor that regulates gene expression in the kidney, liver, pancreas, and other epithelial organs. Mutations of HNF-1beta lead to a syndrome of inherited renal cysts and diabetes and are also a common cause of sporadic renal dysplasia. The full complement of target genes responsible for the functions of HNF-1beta, however, is incompletely defined. Using a functional genomics approach involving chromatin immunoprecipitation and promoter arrays, combined with gene expression profiling, we found that an HNF-1beta target gene in the kidney is kinesin family member 12 (Kif12), a gene previously identified as a candidate modifier gene in the cpk mouse model of polycystic kidney disease. Mutations of HNF-1beta inhibited Kif12 transcription in both cultured cells and knockout mice by altering co-factor recruitment and histone modification. Because kinesin-12 family members participate in orienting cell division, downregulation of Kif12 may underlie the abnormal planar cell polarity observed in cystic kidney diseases.
Figures



References
-
- Igarashi P, Shao X, McNally BT, Hiesberger T: Roles of HNF-1β in kidney development and congenital cystic diseases. Kidney Int 68: 1944–1947, 2005 - PubMed
-
- Hiesberger T, Shao X, Gourley E, Reimann A, Pontoglio M, Igarashi P: Role of the hepatocyte nuclear factor-1β (HNF-1β) C-terminal domain in Pkhd1 (ARPKD) gene transcription and renal cystogenesis. J Biol Chem 280: 10578–10586, 2005 - PubMed
-
- Blumenfeld M, Maury M, Chouard T, Yaniv M, Condamine H: Hepatic nuclear factor 1 (HNF1) shows a wider distribution than products of its known target genes in developing mouse. Development 113: 589–599, 1991 - PubMed
-
- Ott MO, Rey-Campos J, Cereghini S, Yaniv M: vHNF1 is expressed in epithelial cells of distinct embryonic origin during development and precedes HNF1 expression. Mech Dev 36: 47–58, 1991 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

