Assessment of iothalamate plasma clearance: duration of study affects quality of GFR
- PMID: 19005012
- PMCID: PMC2615714
- DOI: 10.2215/CJN.03720708
Assessment of iothalamate plasma clearance: duration of study affects quality of GFR
Abstract
Background and objectives: Measurement of GFR is important for the management of chronic kidney disease (CKD). Although bolus administration of radiocontrast agents is commonly used to measure GFR, the optimal duration of sampling to assess their plasma clearance is unknown. The purpose of this study was to evaluate whether the duration of plasma sampling influences precision and estimation of GFR.
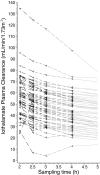
Design, setting, participants, & measurements: GFR was measured by sampling plasma 12 times over 5 h in 56 patients with CKD (mean age 64 yr, 98% men, 79% Caucasian, 34% diabetics, estimated GFR 31.8 +/- 14.2 ml/min/1.73 m(2)). In a subset of 12 patients we measured GFR by sampling plasma 17 times over 10 h.
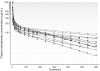
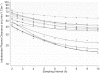
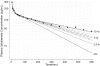
Results: Short sampling intervals considerably overestimated GFR measured using total plasma iothalamate clearance, especially in larger patients. In the higher estimated GFR group (>30 ml/min/1.73 m(2)), the 5-h GFR was 17% higher and 2-h GFR 54% higher compared with the 10-h GFR, which averaged 40.3 ml/min/1.73 m(2). In the lower estimated GFR group (<30 ml/min/1.73 m(2)), the 5-h GFR was 36% higher and 2-h GFR 126% higher compared with the 10-h GFR, which averaged 22.2 ml/min/1.73 m(2). Short sampling duration also reduced the precision of the estimated GFR from 1.67% for 10-h GFR, to 3.48% for 5-h GFR, and to 7.07% for 2-h GFR.
Conclusions: GFR measured over a longer duration with multiple plasma samples spanning the distribution and elimination phases may improve precision and provide a better measure of renal function.
Figures
References
-
- Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G: National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 - PubMed
-
- Levey AS, Greene T, Schluchter MD, Cleary PA, Teschan PE, Lorenz RA, Molitch ME, Mitch WE, Siebert C, Hall PM: Glomerular filtration rate measurements in clinical trials. Modification of Diet in Renal Disease Study Group and the Diabetes Control and Complications Trial Research Group. J Am Soc Nephrol 4: 1159–1171, 1993 - PMC - PubMed
-
- Erley CM, Bader BD, Berger ED, Vochazer A, Jorzik JJ, Dietz K, Risler T: Plasma clearance of iodine contrast media as a measure of glomerular filtration rate in critically ill patients. Crit Care Med 29: 1544–1550, 2001 - PubMed
-
- Holliday MA, Heilbron D, Al-Uzri A, Hidayat J, Uauy R, Conley S, Reisch J, Hogg RJ: Serial measurements of GFR in infants using the continuous iothalamate infusion technique. University of California San Francisco (UCSF) and Southwest Pediatric Nephrology Study Group (SPNSG). Kidney Int 43: 893–898, 1993 - PubMed
-
- Al-Uzri A, Holliday MA, Gambertoglio JG, Schambelan M, Kogan BA, Don BR: An accurate practical method for estimating GFR in clinical studies using a constant subcutaneous infusion. Kidney Int 41: 1701–1706, 1992 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

