Influence of promoter, gene copy number, and preexisting immunity on humoral and cellular responses to a vectored antigen delivered by a Salmonella enterica vaccine
- PMID: 19005022
- PMCID: PMC2620664
- DOI: 10.1128/CVI.00253-08
Influence of promoter, gene copy number, and preexisting immunity on humoral and cellular responses to a vectored antigen delivered by a Salmonella enterica vaccine
Abstract
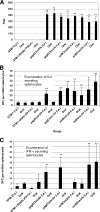
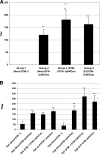
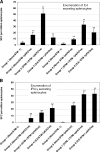
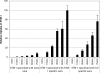
Attenuated Salmonella strains are currently in production as vaccines for protection of animals against salmonellosis. Such commercial strains offer the potential to deliver heterologous antigen to protect animals against other diseases. One vaccine strain, attenuated Salmonella enterica serovar Typhimurium (STM-1), was tested for the ability to deliver ovalbumin and to induce immune responses in mice. Two vaccine trials were performed testing the influence of promoter choice, the location of the encoding DNA (plasmid or chromosome), and the effect of preexisting homologous or heterologous immunity. The results demonstrated that humoral and T-cell responses were induced from either of two promoters, from either the plasmid or the chromosome, and that preexposure to the empty homologous vector, STM-1, or the heterologous vector, S. enterica serovar Enteritidis, had no detrimental effect on subsequent antigen-specific responses. In the case of homologous preexposure, responses were generally greater, and this was correlated with an increased uptake of Salmonella by macrophages in vitro after opsonization with immune sera.
Figures
Similar articles
-
Attenuating gene expression (AGE) for vaccine development.Virulence. 2013 Jul 1;4(5):384-90. doi: 10.4161/viru.24886. Epub 2013 May 7. Virulence. 2013. PMID: 23652809 Free PMC article. Review.
-
Construction, characterization, and immunogenicity of an attenuated Salmonella enterica serovar typhimurium pgtE vaccine expressing fimbriae with integrated viral epitopes from the spiC promoter.Infect Immun. 2003 Aug;71(8):4664-73. doi: 10.1128/IAI.71.8.4664-4673.2003. Infect Immun. 2003. PMID: 12874347 Free PMC article.
-
Refined live attenuated Salmonella enterica serovar Typhimurium and Enteritidis vaccines mediate homologous and heterologous serogroup protection in mice.Infect Immun. 2015 Dec;83(12):4504-12. doi: 10.1128/IAI.00924-15. Epub 2015 Sep 8. Infect Immun. 2015. PMID: 26351285 Free PMC article.
-
Effect of expression level on immune responses to recombinant oral Salmonella enterica serovar Typhimurium vaccines.Vaccine. 2009 May 5;27(20):2707-11. doi: 10.1016/j.vaccine.2009.02.072. Epub 2009 Mar 3. Vaccine. 2009. PMID: 19428883 Free PMC article.
-
Potential therapeutic anti-tumor effect of a Salmonella-based vaccine.Hum Vaccin Immunother. 2013 Aug;9(8):1654-60. doi: 10.4161/hv.24917. Epub 2013 Jun 3. Hum Vaccin Immunother. 2013. PMID: 23733040 Free PMC article. Review.
Cited by
-
Attenuated Listeria monocytogenes: a powerful and versatile vector for the future of tumor immunotherapy.Front Cell Infect Microbiol. 2014 May 12;4:51. doi: 10.3389/fcimb.2014.00051. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24860789 Free PMC article. Review.
-
Attenuating gene expression (AGE) for vaccine development.Virulence. 2013 Jul 1;4(5):384-90. doi: 10.4161/viru.24886. Epub 2013 May 7. Virulence. 2013. PMID: 23652809 Free PMC article. Review.
-
Live-Attenuated Salmonella-Based Oral Vaccine Candidates Expressing PCV2d Cap and Rep by Novel Expression Plasmids as a Vaccination Strategy for Mucosal and Systemic Immune Responses against PCV2d.Vaccines (Basel). 2023 Nov 28;11(12):1777. doi: 10.3390/vaccines11121777. Vaccines (Basel). 2023. PMID: 38140182 Free PMC article.
-
Improved delivery of the OVA-CD4 peptide to T helper cells by polymeric surface display on Salmonella.Microb Cell Fact. 2014 Jun 4;13:80. doi: 10.1186/1475-2859-13-80. Microb Cell Fact. 2014. PMID: 24898796 Free PMC article.
-
Induction of complex immune responses and strong protection against retrovirus challenge by adenovirus-based immunization depends on the order of vaccine delivery.Retrovirology. 2017 Feb 6;14(1):8. doi: 10.1186/s12977-017-0336-7. Retrovirology. 2017. PMID: 28166802 Free PMC article.
References
-
- Alderton, M. R., K. J. Fahey, and P. J. Coloe. 1991. Humoral responses and salmonellosis protection in chickens given a vitamin-dependent Salmonella typhimurium mutant. Avian Dis. 35435-442. - PubMed
-
- al-Ramadi, B. K., M. J. Fernandez-Cabezudo, A. Ullah, H. El-Hasasna, and R. A. Flavell. 2006. CD154 is essential for protective immunity in experimental salmonella infection: evidence for a dual role in innate and adaptive immune responses. J. Immunol. 176496-506. - PubMed
-
- Ashby, D., I. Leduc, W. Lauzon, B. Craig Lee, N. Singhal, and D. W. Cameron. 2005. Attenuated Salmonella typhimurium SL3261 as a vaccine vector for recombinant antigen in rabbits. J. Immunol. Methods 299153-164. - PubMed
-
- Atkins, H. S., M. Morton, K. F. Griffin, M. G. Stokes, J. P. Nataro, and R. W. Titball. 2006. Recombinant Salmonella vaccines for biodefence. Vaccine 242710-2717. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

