Light-induced rescue of breathing after spinal cord injury
- PMID: 19005051
- PMCID: PMC2615537
- DOI: 10.1523/JNEUROSCI.3378-08.2008
Light-induced rescue of breathing after spinal cord injury
Abstract
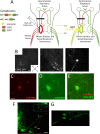
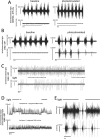
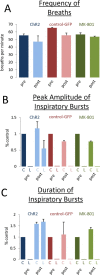
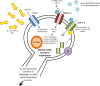
Paralysis is a major consequence of spinal cord injury (SCI). After cervical SCI, respiratory deficits can result through interruption of descending presynaptic inputs to respiratory motor neurons in the spinal cord. Expression of channelrhodopsin-2 (ChR2) and photostimulation in neurons affects neuronal excitability and produces action potentials without any kind of presynaptic inputs. We hypothesized that after transducing spinal neurons in and around the phrenic motor pool to express ChR2, photostimulation would restore respiratory motor function in cervical SCI adult animals. Here we show that light activation of ChR2-expressing animals was sufficient to bring about recovery of respiratory diaphragmatic motor activity. Furthermore, robust rhythmic activity persisted long after photostimulation had ceased. This recovery was accomplished through a form of respiratory plasticity and spinal adaptation which is NMDA receptor dependent. These data suggest a novel, minimally invasive therapeutic avenue to exercise denervated circuitry and/or restore motor function after SCI.
Figures

References
-
- Bellingham MC. Synaptic inhibition of cat phrenic motoneurons by internal intercostal nerve stimulation. J Neurophysiol. 1999;82:1224–1232. - PubMed
-
- Bertram E. The relevance of kindling for human epilepsy. Epilepsia. 2007;48(Suppl 2):65–74. - PubMed
-
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
