Protein kinase Cdelta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current
- PMID: 19005054
- PMCID: PMC2688726
- DOI: 10.1523/JNEUROSCI.3156-08.2008
Protein kinase Cdelta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current
Abstract
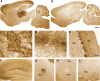
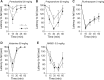
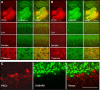
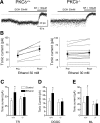
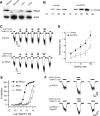
Ethanol alters the distribution and abundance of PKCdelta in neural cell lines. Here we investigated whether PKCdelta also regulates behavioral responses to ethanol. PKCdelta(-/-) mice showed reduced intoxication when administered ethanol and reduced ataxia when administered the nonselective GABA(A) receptor agonists pentobarbital and pregnanolone. However, their response to flunitrazepam was not altered, suggesting that PKCdelta regulates benzodiazepine-insensitive GABA(A) receptors, most of which contain delta subunits and mediate tonic inhibitory currents in neurons. Indeed, the distribution of PKCdelta overlapped with GABA(A) delta subunits in thalamus and hippocampus, and ethanol failed to enhance tonic GABA currents in PKCdelta(-/-) thalamic and hippocampal neurons. Moreover, using an ATP analog-sensitive PKCdelta mutant in mouse L(tk(-)) fibroblasts that express alpha4beta3delta GABA(A) receptors, we found that ethanol enhancement of GABA currents was PKCdelta-dependent. Thus, PKCdelta enhances ethanol intoxication partly through regulation of GABA(A) receptors that contain delta subunits and mediate tonic inhibitory currents. These findings indicate that PKCdelta contributes to a high level of behavioral response to ethanol, which is negatively associated with risk of developing an alcohol use disorder in humans.
Figures

References
-
- Borghese CM, Stórustovu S, Ebert B, Herd MB, Belelli D, Lambert JJ, Marshall G, Wafford KA, Harris RA. The delta subunit of gamma-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316:1360–1368. - PubMed
-
- Bowers BJ, Owen EH, Collins AC, Abeliovich A, Tonegawa S, Wehner JM. Decreased ethanol sensitivity and tolerance development in gamma-protein kinase C null mutant mice is dependent on genetic background. Alcohol Clin Exp Res. 1999;23:387–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
