Group I metabotropic glutamate receptors control metaplasticity of spinal cord learning through a protein kinase C-dependent mechanism
- PMID: 19005059
- PMCID: PMC2628285
- DOI: 10.1523/JNEUROSCI.3098-08.2008
Group I metabotropic glutamate receptors control metaplasticity of spinal cord learning through a protein kinase C-dependent mechanism
Abstract
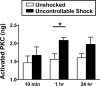
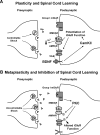
Neurons within the spinal cord can support several forms of plasticity, including response-outcome (instrumental) learning. After a complete spinal transection, experimental subjects are capable of learning to hold the hindlimb in a flexed position (response) if shock (outcome) is delivered to the tibialis anterior muscle when the limb is extended. This response-contingent shock produces a robust learning that is mediated by ionotropic glutamate receptors (iGluRs). Exposure to nociceptive stimuli that are independent of limb position (e.g., uncontrollable shock; peripheral inflammation) produces a long-term (>24 h) inhibition of spinal learning. This inhibition of plasticity in spinal learning is itself a form of plasticity that requires iGluR activation and protein synthesis. Plasticity of plasticity (metaplasticity) in the CNS has been linked to group I metabotropic glutamate receptors (subtypes mGluR1 and mGluR5) and activation of protein kinase C (PKC). The present study explores the role of mGluRs and PKC in the metaplastic inhibition of spinal cord learning using a combination of behavioral, pharmacological, and biochemical techniques. Activation of group I mGluRs was found to be both necessary and sufficient for metaplastic inhibition of spinal learning. PKC was activated by stimuli that inhibit spinal learning, and inhibiting PKC activity restored the capacity for spinal learning. Finally, a PKC inhibitor blocked the metaplastic inhibition of spinal learning produced by a group I mGluR agonist. The data strongly suggest that group I mGluRs control metaplasticity of spinal learning through a PKC-dependent mechanism, providing a potential therapeutic target for promoting use-dependent plasticity after spinal cord injury.
Figures






Similar articles
-
Metaplasticity and behavior: how training and inflammation affect plastic potential within the spinal cord and recovery after injury.Front Neural Circuits. 2014 Sep 8;8:100. doi: 10.3389/fncir.2014.00100. eCollection 2014. Front Neural Circuits. 2014. PMID: 25249941 Free PMC article. Review.
-
Group I metabotropic glutamate receptor (mGluR)-dependent long-term depression mediated via p38 mitogen-activated protein kinase is inhibited by previous high-frequency stimulation and activation of mGluRs and protein kinase C in the rat dentate gyrus in vitro.J Neurosci. 2002 Jul 15;22(14):6121-8. doi: 10.1523/JNEUROSCI.22-14-06121.2002. J Neurosci. 2002. PMID: 12122073 Free PMC article.
-
Alterations in GluR2 AMPA receptor phosphorylation at serine 880 following group I metabotropic glutamate receptor stimulation in the rat dorsal striatum.J Neurosci Res. 2010 Apr;88(5):992-9. doi: 10.1002/jnr.22275. J Neurosci Res. 2010. PMID: 19908285
-
Tetanic stimulation and metabotropic glutamate receptor agonists modify synaptic responses and protein kinase activity in rat auditory cortex.Brain Res. 2001 Mar 16;894(2):218-32. doi: 10.1016/s0006-8993(01)02052-2. Brain Res. 2001. PMID: 11251195
-
Mechanisms of synaptic depression triggered by metabotropic glutamate receptors.Cell Mol Life Sci. 2008 Sep;65(18):2913-23. doi: 10.1007/s00018-008-8263-3. Cell Mol Life Sci. 2008. PMID: 18712277 Free PMC article. Review.
Cited by
-
Maladaptive spinal plasticity opposes spinal learning and recovery in spinal cord injury.Front Physiol. 2012 Oct 10;3:399. doi: 10.3389/fphys.2012.00399. eCollection 2012. Front Physiol. 2012. PMID: 23087647 Free PMC article.
-
Time-dependent cross talk between spinal serotonin 5-HT2A receptor and mGluR1 subserves spinal hyperexcitability and neuropathic pain after nerve injury.J Neurosci. 2012 Sep 26;32(39):13568-81. doi: 10.1523/JNEUROSCI.1364-12.2012. J Neurosci. 2012. PMID: 23015446 Free PMC article.
-
Spinal activation of protein kinase C elicits phrenic motor facilitation.Respir Physiol Neurobiol. 2018 Oct;256:36-42. doi: 10.1016/j.resp.2017.10.007. Epub 2017 Nov 2. Respir Physiol Neurobiol. 2018. PMID: 29081358 Free PMC article.
-
The Spinal Cord, Not to Be Forgotten: the Final Common Path for Development, Training and Recovery of Motor Function.Perspect Behav Sci. 2018 Nov 13;41(2):369-393. doi: 10.1007/s40614-018-00177-9. eCollection 2018 Nov. Perspect Behav Sci. 2018. PMID: 31976401 Free PMC article.
-
Metaplasticity and behavior: how training and inflammation affect plastic potential within the spinal cord and recovery after injury.Front Neural Circuits. 2014 Sep 8;8:100. doi: 10.3389/fncir.2014.00100. eCollection 2014. Front Neural Circuits. 2014. PMID: 25249941 Free PMC article. Review.
References
-
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. - PubMed
-
- Abraham WC, Tate WP. Metaplasticity: a new vista across the field of synaptic plasticity. Prog Neurobiol. 1997;52:303–323. - PubMed
-
- Agrawal SK, Theriault E, Fehlings MG. Role of group I metabotropic glutamate receptors in traumatic spinal cord white matter injury. J Neurotrauma. 1998;15:929–941. - PubMed
-
- Ali DW, Salter MW. NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr Opin Neurobiol. 2001;11:336–342. - PubMed
-
- Aniksztejn L, Otani S, Ben Ari Y. Quisqualate metabotropic receptors modulate NMDA currents and facilitate induction of long-term potentiation through protein kinase C. Eur J Neurosci. 1992;4:500–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
