Maladaptive homeostatic plasticity in a rodent model of central pain syndrome: thalamic hyperexcitability after spinothalamic tract lesions
- PMID: 19005061
- PMCID: PMC2627563
- DOI: 10.1523/JNEUROSCI.3296-08.2008
Maladaptive homeostatic plasticity in a rodent model of central pain syndrome: thalamic hyperexcitability after spinothalamic tract lesions
Abstract
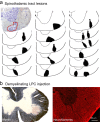
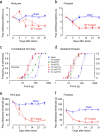
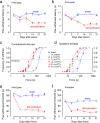
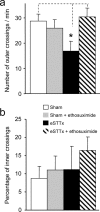
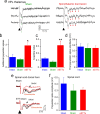
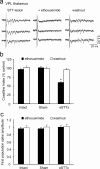
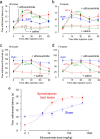
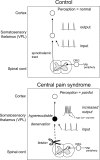
Central pain syndrome (CPS) is defined as pain associated with a lesion of the CNS and is a common consequence of spinal cord injuries. We generated a rodent model of CPS by making unilateral electrolytic or demyelinating lesions centered on the spinothalamic tract in rats. Thermal hyperalgesia and mechanical allodynia occurred in both hind paws and forepaws by 7 d postlesion and were maintained >31 d. Field potentials in the ventral posterior lateral nucleus (VPL) in thalamic brain slices from lesioned animals displayed an increased probability of burst responses. Ethosuximide, a T-type calcium channel blocker, eliminated busting in lesioned thalamic slices and attenuated lesion-induced hyperalgesia and allodynia. We conclude that CPS in this model results from an increase in the excitability of thalamic nuclei that have lost normal ascending inputs as the result of a spinal cord injury and suggest that ethosuximide will relieve human CPS by restoring normal thalamic excitability.
Figures
Similar articles
-
Functional plasticity in primate somatosensory thalamus following chronic lesion of the ventral lateral spinal cord.Neuroscience. 2000;101(2):393-401. doi: 10.1016/s0306-4522(00)00368-7. Neuroscience. 2000. PMID: 11074162
-
Role of microglia and astrocyte in central pain syndrome following electrolytic lesion at the spinothalamic tract in rats.J Mol Neurosci. 2013 Mar;49(3):470-9. doi: 10.1007/s12031-012-9840-3. Epub 2012 Jun 22. J Mol Neurosci. 2013. PMID: 22722907
-
Estradiol attenuates spinal cord injury-related central pain by decreasing glutamate levels in thalamic VPL nucleus in male rats.Metab Brain Dis. 2014 Sep;29(3):763-70. doi: 10.1007/s11011-014-9570-z. Epub 2014 May 31. Metab Brain Dis. 2014. PMID: 24879046
-
Allodynia and hyperalgesia within dermatomes caudal to a spinal cord injury in primates and rodents.Prog Brain Res. 2000;129:411-28. doi: 10.1016/S0079-6123(00)29032-8. Prog Brain Res. 2000. PMID: 11098708 Review. No abstract available.
-
Neuroscience of the human thalamus related to acute pain and chronic "thalamic" pain.J Neurophysiol. 2024 Dec 1;132(6):1756-1778. doi: 10.1152/jn.00065.2024. Epub 2024 Oct 16. J Neurophysiol. 2024. PMID: 39412562 Review.
Cited by
-
Reduced GABAergic transmission in the ventrobasal thalamus contributes to thermal hyperalgesia in chronic inflammatory pain.Sci Rep. 2017 Feb 2;7:41439. doi: 10.1038/srep41439. Sci Rep. 2017. PMID: 28150719 Free PMC article.
-
How is chronic pain related to sympathetic dysfunction and autonomic dysreflexia following spinal cord injury?Auton Neurosci. 2018 Jan;209:79-89. doi: 10.1016/j.autneu.2017.01.006. Epub 2017 Jan 27. Auton Neurosci. 2018. PMID: 28161248 Free PMC article. Review.
-
Post-translational modification of cortical GluA receptors in rodents following spinal cord lesion.Neuroscience. 2016 Mar 1;316:122-9. doi: 10.1016/j.neuroscience.2015.12.038. Epub 2015 Dec 24. Neuroscience. 2016. PMID: 26724583 Free PMC article.
-
Association of pain and CNS structural changes after spinal cord injury.Sci Rep. 2016 Jan 6;6:18534. doi: 10.1038/srep18534. Sci Rep. 2016. PMID: 26732942 Free PMC article.
-
Zona incerta: a role in central pain.J Neurophysiol. 2009 Jul;102(1):181-91. doi: 10.1152/jn.00152.2009. Epub 2009 Apr 29. J Neurophysiol. 2009. PMID: 19403748 Free PMC article.
References
-
- Berić A, Dimitrijević MR, Lindblom U. Central dysesthesia syndrome in spinal cord injury patients. Pain. 1988;34:109–116. - PubMed
-
- Bonica JJ. History of pain concepts and pain therapy. Mt Sinai J Med. 1991;58:191–202. - PubMed
-
- Cannavero S, Bonicalzi V. Central pain syndrome: pathophysiology, diagnosis and management. Cambridge, UK: Cambridge UP; 2007.
-
- Coulter DA, Huguenard JR, Prince DA. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann Neurol. 1989;25:582–593. - PubMed
-
- Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
