Induced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cell neurons via activated T-cell-derived fas-ligand
- PMID: 19005073
- PMCID: PMC2683273
- DOI: 10.1523/JNEUROSCI.3200-08.2008
Induced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cell neurons via activated T-cell-derived fas-ligand
Abstract
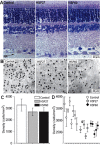
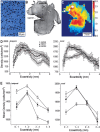
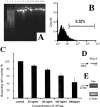
Glaucomatous optic neuropathy causes blindness through the degeneration of retinal ganglion cells (RGCs) and their axons, which comprise the optic nerve. Glaucoma traditionally is associated with elevated intraocular pressure, but often occurs or may progress with intraocular pressure in the normal range. Like other diseases of the CNS, a subset of glaucoma has been proposed to involve an autoimmune component to help explain the loss of RGCs in the absence of elevated intraocular pressure. One hypothesis involves heat shock proteins (HSPs), because increased serum levels of HSP autoantibodies are prominent in some glaucoma patients with normal pressures. In the first direct support of this hypothesis, we found that HSP27 and HSP60 immunization in the Lewis rat induced RGC degeneration and axon loss 1-4 months later in vivo in a pattern with similarities to human glaucoma, including topographic specificity of cell loss. Infiltration of increased numbers of T-cells in the retina occurred much earlier, 14-21 d after HSP immunization, and appeared to be transient. In vitro studies found that T-cells activated by HSP immunization induced RGC apoptosis via the release of the inflammatory cytokine FasL, whereas HSP immunization induced activation of microglia cells and upregulation of the FasL receptor in RGCs. In summary, our results suggest that RGC degeneration in glaucoma for selected individuals likely involves failed immunoregulation of the T-cell-RGC axis and is thus a disturbance of both proapoptotic and protective pathways.
Figures




References
-
- Aloisi F. The role of microglia and astrocytes in CNS immune surveillance and immunopathology. Adv Exp Med Biol. 1999;468:123–133. - PubMed
-
- Andersen O, Lygner PE, Bergström T, Andersson M, Vahlne A. Viral infections trigger multiple sclerosis relapses: a prospective seroepidemiological study. J Neurol. 1993;240:417–422. - PubMed
-
- Araie M. Pattern of visual field defects in normal-tension and high-tension glaucoma. Curr Opin Ophthalmol. 1995;6:36–45. - PubMed
-
- Ashwell KW, Holländer H, Streit W, Stone J. The appearance and distribution of microglia in the developing retina of the rat. Vis Neurosci. 1989;2:437–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
