Loss of gamma-secretase function impairs endocytosis of lipoprotein particles and membrane cholesterol homeostasis
- PMID: 19005074
- PMCID: PMC6671640
- DOI: 10.1523/JNEUROSCI.2635-08.2008
Loss of gamma-secretase function impairs endocytosis of lipoprotein particles and membrane cholesterol homeostasis
Abstract
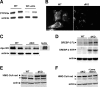
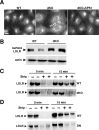
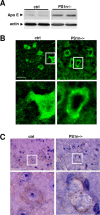
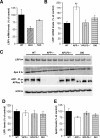
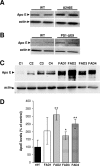
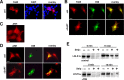
Presenilins (PSs) are components of the gamma-secretase complex that mediates intramembranous cleavage of type I membrane proteins. We show that gamma-secretase is involved in the regulation of cellular lipoprotein uptake. Loss of gamma-secretase function decreased endocytosis of low-density lipoprotein (LDL) receptor. The decreased uptake of lipoproteins led to upregulation of cellular cholesterol biosynthesis by increased expression of CYP51 and enhanced metabolism of lanosterol. Genetic deletion of PS1 or transgenic expression of PS1 mutants that cause early-onset Alzheimer's disease led to accumulation of gamma-secretase substrates and mistargeting of adaptor proteins that regulate endocytosis of the LDL receptor. Consistent with decreased endocytosis of these receptors, PS1 mutant mice have elevated levels of apolipoprotein E in the brain. Thus, these data demonstrate a functional link between two major genetic factors that cause early-onset and late-onset Alzheimer's disease.
Figures

References
-
- Annaert WG, Esselens C, Baert V, Boeve C, Snellings G, Cupers P, Craessaerts K, De Strooper B. Interaction with telencephalin and the amyloid precursor protein predicts a ring structure for presenilins. Neuron. 2001;32:579–589. - PubMed
-
- Bales KR, Dodart JC, DeMattos RB, Holtzman DM, Paul SM. Apolipoprotein E, amyloid, and Alzheimer disease. Mol Interv. 2002;2:363–375. - PubMed
-
- Bentahir M, Nyabi O, Verhamme J, Tolia A, Horré K, Wiltfang J, Esselmann H, De Strooper B. Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms. J Neurochem. 2006;96:732–742. - PubMed
-
- Berger-Sweeney J, McPhie DL, Arters JA, Greenan J, Oster-Granite ML, Neve RL. Impairments in learning and memory accompanied by neurodegeneration in mice transgenic for the carboxyl-terminus of the amyloid precursor protein. Brain Res Mol Brain Res. 1999;66:150–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
