Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity
- PMID: 19005075
- PMCID: PMC2752048
- DOI: 10.1523/JNEUROSCI.2642-08.2008
Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity
Abstract
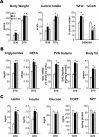
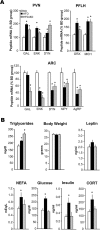
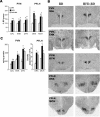
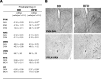
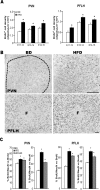
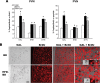
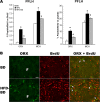
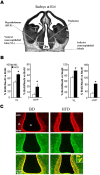
Recent studies in adult and weanling rats show that dietary fat, in close association with circulating lipids, can stimulate expression of hypothalamic peptides involved in controlling food intake and body weight. In the present study, we examined the possibility that a fat-rich diet during pregnancy alters the development of these peptide systems in utero, producing neuronal changes in the offspring that persist postnatally in the absence of the diet and have long-term consequences. The offspring of dams on a high-fat diet (HFD) versus balanced diet (BD), from embryonic day 6 to postnatal day 15 (P15), showed increased expression of orexigenic peptides, galanin, enkephalin, and dynorphin, in the paraventricular nucleus and orexin and melanin-concentrating hormone in the perifornical lateral hypothalamus. The increased density of these peptide-expressing neurons, evident in newborn offspring as well as P15 offspring cross-fostered at birth to dams on the BD, led us to examine events that might be occurring in utero. During gestation, the HFD stimulated the proliferation of neuroepithelial and neuronal precursor cells of the embryonic hypothalamic third ventricle. It also stimulated the proliferation and differentiation of neurons and their migration toward hypothalamic areas where ultimately a greater proportion of the new neurons expressed the orexigenic peptides. This increase in neurogenesis, closely associated with a marked increase in lipids in the blood, may have a role in producing the long-term behavioral and physiological changes observed in offspring after weaning, including an increase in food intake, preference for fat, hyperlipidemia, and higher body weight.
Figures

Similar articles
-
Prenatal exposure to nicotine stimulates neurogenesis of orexigenic peptide-expressing neurons in hypothalamus and amygdala.J Neurosci. 2013 Aug 21;33(34):13600-11. doi: 10.1523/JNEUROSCI.5835-12.2013. J Neurosci. 2013. PMID: 23966683 Free PMC article.
-
Prenatal fat exposure and hypothalamic PPAR β/δ: Possible relationship to increased neurogenesis of orexigenic peptide neurons.Peptides. 2016 May;79:16-26. doi: 10.1016/j.peptides.2016.03.007. Epub 2016 Mar 19. Peptides. 2016. PMID: 27002387 Free PMC article.
-
Increased orexin and melanin-concentrating hormone expression in the perifornical lateral hypothalamus of rats prone to overconsuming a fat-rich diet.Pharmacol Biochem Behav. 2010 Oct;96(4):413-22. doi: 10.1016/j.pbb.2010.06.013. Epub 2010 Jul 1. Pharmacol Biochem Behav. 2010. PMID: 20600243 Free PMC article.
-
Behavioral Feeding Circuit: Dietary Fat-Induced Effects of Inflammatory Mediators in the Hypothalamus.Front Endocrinol (Lausanne). 2020 Nov 26;11:591559. doi: 10.3389/fendo.2020.591559. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33324346 Free PMC article. Review.
-
[A role for orexins and melanin-concentrating hormone in the central regulation of feeding behavior].Nihon Rinsho. 2001 Mar;59(3):427-30. Nihon Rinsho. 2001. PMID: 11268588 Review. Japanese.
Cited by
-
Parental obesity-induced changes in developmental programming.Front Cell Dev Biol. 2022 Oct 7;10:918080. doi: 10.3389/fcell.2022.918080. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36274855 Free PMC article. Review.
-
Development, brain plasticity and reward: early high-fat diet exposure confers vulnerability to obesity-view from the chair.Int J Obes Suppl. 2012 Dec;2(Suppl 2):S3-6. doi: 10.1038/ijosup.2012.14. Epub 2012 Dec 11. Int J Obes Suppl. 2012. PMID: 27152151 Free PMC article.
-
The role of maternal obesity in the risk of neuropsychiatric disorders.Front Neurosci. 2015 Jun 18;9:194. doi: 10.3389/fnins.2015.00194. eCollection 2015. Front Neurosci. 2015. PMID: 26150767 Free PMC article. Review.
-
Neonatal anthropometrics and correlation to childhood obesity--data from the Danish Children's Obesity Clinic.Eur J Pediatr. 2013 Jun;172(6):747-51. doi: 10.1007/s00431-013-1949-z. Epub 2013 Feb 1. Eur J Pediatr. 2013. PMID: 23371390
-
Early High-Fat Diet Exposure Causes Dysregulation of the Orexin and Dopamine Neuronal Populations in Nonhuman Primates.Front Endocrinol (Lausanne). 2018 Sep 10;9:508. doi: 10.3389/fendo.2018.00508. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30258403 Free PMC article.
References
-
- Adams AC, Clapham JC, Wynick D, Speakman JR. Feeding behaviour in galanin knockout mice supports a role of galanin in fat intake and preference. J Neuroendocrinol. 2008;20:199–206. - PubMed
-
- Akabayashi A, Watanabe Y, Wahlestedt C, McEwen BS, Paez X, Leibowitz SF. Hypothalamic neuropeptide Y, its gene expression and receptor activity: relation to circulating corticosterone in adrenalectomized rats. Brain Res. 1994b;665:201–212. - PubMed
-
- Altman J, Bayer SA. Development of the diencephalon in the rat. II. Correlation of the embryonic development of the hypothalamus with the time of origin of its neurons. J Comp Neurol. 1978;182:973–993. - PubMed
-
- Archer ZA, Rayner DV, Barrett P, Balik A, Duncan JS, Moar KM, Mercer JG. Hypothalamic energy balance gene responses in the Sprague-Dawley rat to supplementation of high-energy diet with liquid ensure and subsequent transfer to chow. J Neuroendocrinol. 2005;17:711–719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
