Microtubules in dendritic spine development
- PMID: 19005076
- PMCID: PMC2605155
- DOI: 10.1523/JNEUROSCI.2509-08.2008
Microtubules in dendritic spine development
Abstract
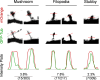
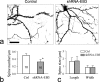
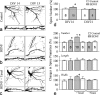
It is generally believed that only the actin cytoskeleton resides in dendritic spines and controls spine morphology and plasticity. Here, we report that microtubules (MTs) are present in spines and that shRNA knockdown of the MT plus-end-binding protein EB3 significantly reduces spine formation. Furthermore, stabilization and inhibition of MTs by low doses of taxol and nocodazole enhance and impair spine formation elicited by BDNF (brain-derived neurotrophic factor), respectively. Therefore, MTs play an important role in the control and regulation of dendritic spine development and plasticity.
Figures

References
-
- Akhmanova A, Hoogenraad CC. Microtubule plus-end-tracking proteins: mechanisms and functions. Curr Opin Cell Biol. 2005;17:47–54. - PubMed
-
- Bacci A, Verderio C, Pravettoni E, Matteoli M. Synaptic and intrinsic mechanisms shape synchronous oscillations in hippocampal neurons in culture. Eur J Neurosci. 1999;11:389–397. - PubMed
-
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
