Crucial role of CB(2) cannabinoid receptor in the regulation of central immune responses during neuropathic pain
- PMID: 19005077
- PMCID: PMC3844839
- DOI: 10.1523/JNEUROSCI.3400-08.2008
Crucial role of CB(2) cannabinoid receptor in the regulation of central immune responses during neuropathic pain
Abstract
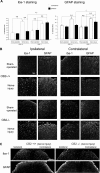
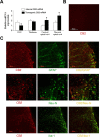
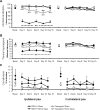
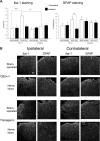
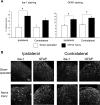
Neuropathic pain is a clinical manifestation of nerve injury difficult to treat even with potent analgesic compounds. Here, we used different lines of genetically modified mice to clarify the role played by CB(2) cannabinoid receptors in the regulation of the central immune responses leading to the development of neuropathic pain. CB(2) knock-out mice and wild-type littermates were exposed to sciatic nerve injury, and both genotypes developed a similar hyperalgesia and allodynia in the ipsilateral paw. Most strikingly, knock-outs also developed a contralateral mirror image pain, associated with an enhanced microglial and astrocytic expression in the contralateral spinal horn. In agreement, hyperalgesia, allodynia, and microglial and astrocytic activation induced by sciatic nerve injury were attenuated in transgenic mice overexpressing CB(2) receptors. These results demonstrate the crucial role of CB(2) cannabinoid receptor in modulating glial activation in response to nerve injury. The enhanced manifestations of neuropathic pain were replicated in irradiated wild-type mice reconstituted with bone marrow cells from CB(2) knock-outs, thus demonstrating the implication of the CB(2) receptor expressed in hematopoietic cells in the development of neuropathic pain at the spinal cord.
Figures


References
-
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. - PMC - PubMed
-
- Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, Reggiani A. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–1538. - PubMed
-
- Bingham B, Jones PG, Uveges AJ, Kotnis S, Lu P, Smith VA, Sun SC, Resnick L, Chlenov M, He Y, Strassle BW, Cummons TA, Piesla MJ, Harrison JE, Whiteside GT, Kennedy JD. Species-specific in vitro pharmacological effects of the cannabinoid receptor 2 (CB2) selective ligand AM1241 and its resolved enantiomers. Br J Pharmacol. 2007;151:1061–1070. - PMC - PubMed
-
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, Glass M, Zimmer A. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2000;396:141–149. - PubMed
-
- Carlisle SJ, Marciano-Cabral F, Staab A, Ludwick C, Cabral GA. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int Immunopharmacol. 2002;2:69–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
