Interferon-gamma is a critical modulator of CB(2) cannabinoid receptor signaling during neuropathic pain
- PMID: 19005078
- PMCID: PMC3844840
- DOI: 10.1523/JNEUROSCI.3402-08.2008
Interferon-gamma is a critical modulator of CB(2) cannabinoid receptor signaling during neuropathic pain
Abstract
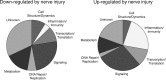
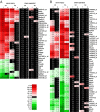
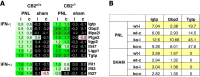
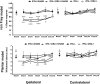
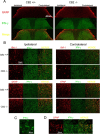
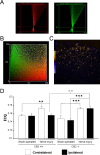
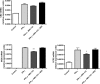
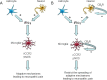
Nerve injuries often lead to neuropathic pain syndrome. The mechanisms contributing to this syndrome involve local inflammatory responses, activation of glia cells, and changes in the plasticity of neuronal nociceptive pathways. Cannabinoid CB(2) receptors contribute to the local containment of neuropathic pain by modulating glial activation in response to nerve injury. Thus, neuropathic pain spreads in mice lacking CB(2) receptors beyond the site of nerve injury. To further investigate the mechanisms leading to the enhanced manifestation of neuropathic pain, we have established expression profiles of spinal cord tissues from wild-type and CB(2)-deficient mice after nerve injury. An enhanced interferon-gamma (IFN-gamma) response was revealed in the absence of CB(2) signaling. Immunofluorescence stainings demonstrated an IFN-gamma production by astrocytes and neurons ispilateral to the nerve injury in wild-type animals. In contrast, CB(2)-deficient mice showed neuronal and astrocytic IFN-gamma immunoreactivity also in the contralateral region, thus matching the pattern of nociceptive hypersensitivity in these animals. Experiments in BV-2 microglia cells revealed that transcriptional changes induced by IFN-gamma in two key elements for neuropathic pain development, iNOS (inducible nitric oxide synthase) and CCR2, are modulated by CB(2) receptor signaling. The most direct support for a functional involvement of IFN-gamma as a mediator of CB(2) signaling was obtained with a double knock-out mouse strain deficient in CB(2) receptors and IFN-gamma. These animals no longer show the enhanced manifestations of neuropathic pain observed in CB(2) knock-outs. These data clearly demonstrate that the CB(2) receptor-mediated control of neuropathic pain is IFN-gamma dependent.
Figures
Similar articles
-
Crucial role of CB(2) cannabinoid receptor in the regulation of central immune responses during neuropathic pain.J Neurosci. 2008 Nov 12;28(46):12125-35. doi: 10.1523/JNEUROSCI.3400-08.2008. J Neurosci. 2008. PMID: 19005077 Free PMC article.
-
Interleukin-18-mediated microglia/astrocyte interaction in the spinal cord enhances neuropathic pain processing after nerve injury.J Neurosci. 2008 Nov 26;28(48):12775-87. doi: 10.1523/JNEUROSCI.3512-08.2008. J Neurosci. 2008. PMID: 19036970 Free PMC article.
-
The cannabinoid CB₂ receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain.Eur Neuropsychopharmacol. 2014 Apr;24(4):608-20. doi: 10.1016/j.euroneuro.2013.10.008. Epub 2013 Oct 22. Eur Neuropsychopharmacol. 2014. PMID: 24210682
-
Activated microglia in the spinal cord underlies diabetic neuropathic pain.Eur J Pharmacol. 2014 Apr 5;728:59-66. doi: 10.1016/j.ejphar.2014.01.057. Epub 2014 Feb 6. Eur J Pharmacol. 2014. PMID: 24508519 Review.
-
Neuropathic pain and spinal microglia: a big problem from molecules in "small" glia.Trends Neurosci. 2005 Feb;28(2):101-7. doi: 10.1016/j.tins.2004.12.002. Trends Neurosci. 2005. PMID: 15667933 Review.
Cited by
-
Allosteric modulation of G protein-coupled receptors as a novel therapeutic strategy in neuropathic pain.Acta Pharm Sin B. 2024 Jan;14(1):67-86. doi: 10.1016/j.apsb.2023.07.020. Epub 2023 Jul 21. Acta Pharm Sin B. 2024. PMID: 38239234 Free PMC article. Review.
-
Crosstalk between endocannabinoid and immune systems: a potential dysregulation in depression?Psychopharmacology (Berl). 2016 May;233(9):1591-604. doi: 10.1007/s00213-015-4105-9. Epub 2015 Oct 20. Psychopharmacology (Berl). 2016. PMID: 26483037 Free PMC article. Review.
-
Confound, Cause, or Cure: The Effect of Cannabinoids on HIV-Associated Neurological Sequelae.Viruses. 2021 Jun 26;13(7):1242. doi: 10.3390/v13071242. Viruses. 2021. PMID: 34206839 Free PMC article. Review.
-
Involvement of interferon gamma signaling in spinal trigeminal caudal subnucleus astrocyte in orofacial neuropathic pain in rats with infraorbital nerve injury.Mol Pain. 2023 Jan-Dec;19:17448069231222403. doi: 10.1177/17448069231222403. Mol Pain. 2023. PMID: 38073236 Free PMC article.
-
Spinal cord injury induced neuropathic pain: Molecular targets and therapeutic approaches.Metab Brain Dis. 2015 Jun;30(3):645-58. doi: 10.1007/s11011-014-9642-0. Epub 2015 Jan 15. Metab Brain Dis. 2015. PMID: 25588751 Review.
References
-
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. - PMC - PubMed
-
- Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. - PubMed
-
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, Glass M, Zimmer A. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2000;396:141–149. - PubMed
-
- Cannella B, Raine CS. Multiple sclerosis: cytokine receptors on oligodendrocytes predict innate regulation. Ann Neurol. 2004;55:46–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
