A relaxed specificity in interchain disulfide bond formation characterizes the assembly of a low-molecular-weight glutenin subunit in the endoplasmic reticulum
- PMID: 19005088
- PMCID: PMC2613710
- DOI: 10.1104/pp.108.127761
A relaxed specificity in interchain disulfide bond formation characterizes the assembly of a low-molecular-weight glutenin subunit in the endoplasmic reticulum
Abstract
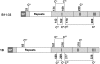
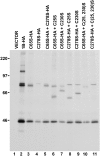
Wheat (Triticum spp.) grains contain large protein polymers constituted by two main classes of polypeptides: the high-molecular-weight glutenin subunits and the low-molecular-weight glutenin subunits (LMW-GS). These polymers are among the largest protein molecules known in nature and are the main determinants of the superior technological properties of wheat flours. However, little is known about the mechanisms controlling the assembly of the different subunits and the way they are arranged in the final polymer. Here, we have addressed these issues by analyzing the formation of interchain disulfide bonds between identical and different LMW-GS and by studying the assembly of mutants lacking individual intrachain disulfides. Our results indicate that individual cysteine residues that remain available for disulfide bond formation in the folded monomer can form interchain disulfide bonds with a variety of different cysteine residues present in a companion subunit. These results imply that the coordinated expression of many different LMW-GS in wheat endosperm cells can potentially lead to the formation of a large set of distinct polymeric structures, in which subunits can be arranged in different configurations. In addition, we show that not all intrachain disulfide bonds are necessary for the generation of an assembly-competent structure and that the retention of a LMW-GS in the early secretory pathway is not dependent on polymer formation.
Figures







References
-
- Altschuler Y, Galili G (1994) Role of conserved cysteines of a wheat gliadin in its transport and assembly into protein bodies in Xenopus oocytes. J Biol Chem 269 6677–82 - PubMed
-
- Ceriotti A, Vitale A, Paris N, Frigerio L, Neuhaus J-M, Hillmer S, Robinson DG (2003) Plant cell biology. In J Davey, JM Lord, eds, Essential Cell Biology, Vol 1. Oxford Univesity Press, Oxford, pp 133–161
-
- Dixon DP, Van Lith M, Edwards R, Benham A (2003) Cloning and initial characterization of the Arabidopsis thaliana endoplasmic reticulum oxidoreductins. Antioxid Redox Signal 5 389–96 - PubMed
-
- D'Ovidio R, Masci S (2004) The low-molecular-weight glutenin subunits of wheat gluten. J Cereal Sci 39 321–339
-
- D'Ovidio R, Simeone M, Masci S, Porceddu E (1997) Molecular characterization of a LMW-GS gene located on chromosome 1B and the development of primers specific for the Glu-B3 complex locus in durum wheat. Theor Appl Genet 95 1119–1126
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

