CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression
- PMID: 19005183
- PMCID: PMC2628380
- DOI: 10.1182/blood-2008-03-145219
CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression
Abstract
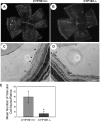
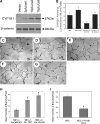
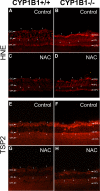
Reactive species derived from cell oxygenation processes play an important role in vascular homeostasis and the pathogenesis of many diseases including retinopathy of prematurity. We show that CYP1B1-deficient (CYP1B1(-/-)) mice fail to elicit a neovascular response during oxygen-induced ischemic retinopathy. In addition, the retinal endothelial cells (ECs) prepared from CYP1B1(-/-) mice are less adherent, less migratory, and fail to undergo capillary morphogenesis. These aberrant cellular responses were completely reversed when oxygen levels were lowered or an antioxidant added. CYP1B1(-/-) ECs exhibited increased oxidative stress and expressed increased amounts of the antiangiogenic factor thrombospondin-2 (TSP2). Increased lipid peroxidation and TSP2 were both observed in retinas from CYP1B1(-/-) mice and were reversed by administration of an antioxidant. Reexpression of CYP1B1 in CYP1B1(-/-) ECs resulted in down-regulation of TSP2 expression and restoration of capillary morphogenesis. A TSP2 knockdown in CYP1B1(-/-) ECs also restored capillary morphogenesis. Thus, CYP1B1 metabolizes cell products that modulate intracellular oxidative stress, which enhances production of TSP2, an inhibitor of EC migration and capillary morphogenesis. Evidence is presented that similar changes occur in retinal endothelium in vivo to limit neovascularization.
Figures
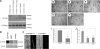


Similar articles
-
CYP1B1 and endothelial nitric oxide synthase combine to sustain proangiogenic functions of endothelial cells under hyperoxic stress.Am J Physiol Cell Physiol. 2010 Mar;298(3):C665-78. doi: 10.1152/ajpcell.00153.2009. Epub 2009 Dec 23. Am J Physiol Cell Physiol. 2010. PMID: 20032512 Free PMC article.
-
Cyp1B1 expression promotes angiogenesis by suppressing NF-κB activity.Am J Physiol Cell Physiol. 2013 Dec 1;305(11):C1170-84. doi: 10.1152/ajpcell.00139.2013. Epub 2013 Oct 2. Am J Physiol Cell Physiol. 2013. PMID: 24088896 Free PMC article.
-
Cyp1b1-deficient retinal astrocytes are more proliferative and migratory and are protected from oxidative stress and inflammation.Am J Physiol Cell Physiol. 2019 Jun 1;316(6):C767-C781. doi: 10.1152/ajpcell.00021.2019. Epub 2019 Mar 20. Am J Physiol Cell Physiol. 2019. PMID: 30892936 Free PMC article.
-
Cytochrome P450 1B1: A Key Regulator of Ocular Iron Homeostasis and Oxidative Stress.Cells. 2022 Sep 20;11(19):2930. doi: 10.3390/cells11192930. Cells. 2022. PMID: 36230892 Free PMC article. Review.
-
New insights into the retinal circulation: inflammatory lipid mediators in ischemic retinopathy.Prostaglandins Leukot Essent Fatty Acids. 2005 May;72(5):301-25. doi: 10.1016/j.plefa.2005.02.004. Prostaglandins Leukot Essent Fatty Acids. 2005. PMID: 15850712 Review.
Cited by
-
CYP1B1 and endothelial nitric oxide synthase combine to sustain proangiogenic functions of endothelial cells under hyperoxic stress.Am J Physiol Cell Physiol. 2010 Mar;298(3):C665-78. doi: 10.1152/ajpcell.00153.2009. Epub 2009 Dec 23. Am J Physiol Cell Physiol. 2010. PMID: 20032512 Free PMC article.
-
Aryl hydrocarbon receptor-dependent upregulation of Cyp1b1 by TCDD and diesel exhaust particles in rat brain microvessels.Fluids Barriers CNS. 2011 Aug 25;8:23. doi: 10.1186/2045-8118-8-23. Fluids Barriers CNS. 2011. PMID: 21867498 Free PMC article.
-
Matricellular protein thrombospondins: influence on ocular angiogenesis, wound healing and immuneregulation.Curr Eye Res. 2014 Aug;39(8):759-74. doi: 10.3109/02713683.2013.877936. Epub 2014 Feb 21. Curr Eye Res. 2014. PMID: 24559320 Free PMC article. Review.
-
Mechanisms of nanosized titanium dioxide-induced testicular oxidative stress and apoptosis in male mice.Part Fibre Toxicol. 2014 Sep 12;11:47. doi: 10.1186/s12989-014-0047-3. Part Fibre Toxicol. 2014. Retraction in: Part Fibre Toxicol. 2015 Jul 14;12:23. doi: 10.1186/s12989-015-0098-0. PMID: 25209749 Free PMC article. Retracted.
-
Inhibition of Rho Kinase Induces Antioxidative Molecules and Suppresses Reactive Oxidative Species in Trabecular Meshwork Cells.J Ophthalmol. 2017;2017:7598140. doi: 10.1155/2017/7598140. Epub 2017 Jul 19. J Ophthalmol. 2017. PMID: 28804648 Free PMC article.
References
-
- Campochiaro PA. Molecular targets for retinal vascular diseases. J Cell Physiol. 2007;210:575–581. - PubMed
-
- Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. - PubMed
-
- Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 2001;50:1938–1942. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

