Polarized traffic of LRP1 involves AP1B and SNX17 operating on Y-dependent sorting motifs in different pathways
- PMID: 19005208
- PMCID: PMC2613102
- DOI: 10.1091/mbc.e08-08-0805
Polarized traffic of LRP1 involves AP1B and SNX17 operating on Y-dependent sorting motifs in different pathways
Abstract
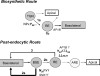
Low-density lipoprotein receptor-related protein 1 (LRP1) is an endocytic recycling receptor with two cytoplasmic tyrosine-based basolateral sorting signals. Here we show that during biosynthetic trafficking LRP1 uses AP1B adaptor complex to move from a post-TGN recycling endosome (RE) to the basolateral membrane. Then it recycles basolaterally from the basolateral sorting endosome (BSE) involving recognition by sorting nexin 17 (SNX17). In the biosynthetic pathway, Y(29) but not N(26) from a proximal NPXY directs LRP1 basolateral sorting from the TGN. A N(26)A mutant revealed that this NPXY motif recognized by SNX17 is required for the receptor's exit from BSE. An endocytic Y(63)ATL(66) motif also functions in basolateral recycling, in concert with an additional endocytic motif (LL(86,87)), by preventing LRP1 entry into the transcytotic apical pathway. All this sorting information operates similarly in hippocampal neurons to mediate LRP1 somatodendritic distribution regardless of the absence of AP1B in neurons. LRP1 basolateral distribution results then from spatially and temporally segregation steps mediated by recognition of distinct tyrosine-based motifs. We also demonstrate a novel function of SNX17 in basolateral/somatodendritic recycling from a different compartment than AP1B endosomes.
Figures









Similar articles
-
Clathrin and AP1B: key roles in basolateral trafficking through trans-endosomal routes.FEBS Lett. 2009 Dec 3;583(23):3784-95. doi: 10.1016/j.febslet.2009.10.050. Epub 2009 Oct 23. FEBS Lett. 2009. PMID: 19854182 Free PMC article. Review.
-
A sorting nexin 17-binding domain within the LRP1 cytoplasmic tail mediates receptor recycling through the basolateral sorting endosome.Traffic. 2013 Jul;14(7):823-38. doi: 10.1111/tra.12076. Epub 2013 May 8. Traffic. 2013. PMID: 23593972 Free PMC article.
-
Sorting nexin 17 (SNX17) links endosomal sorting to Eps15 homology domain protein 1 (EHD1)-mediated fission machinery.J Biol Chem. 2020 Mar 20;295(12):3837-3850. doi: 10.1074/jbc.RA119.011368. Epub 2020 Feb 10. J Biol Chem. 2020. PMID: 32041776 Free PMC article.
-
Antibody to AP1B adaptor blocks biosynthetic and recycling routes of basolateral proteins at recycling endosomes.Mol Biol Cell. 2007 Dec;18(12):4872-84. doi: 10.1091/mbc.e07-06-0563. Epub 2007 Sep 19. Mol Biol Cell. 2007. PMID: 17881725 Free PMC article.
-
Protein sorting in the Golgi complex: shifting paradigms.Biochim Biophys Acta. 2005 Jul 10;1744(3):455-64. doi: 10.1016/j.bbamcr.2005.04.007. Biochim Biophys Acta. 2005. PMID: 15927284 Review.
Cited by
-
Cellular prion protein participates in amyloid-β transcytosis across the blood-brain barrier.J Cereb Blood Flow Metab. 2012 Apr;32(4):628-32. doi: 10.1038/jcbfm.2012.7. Epub 2012 Feb 1. J Cereb Blood Flow Metab. 2012. PMID: 22293988 Free PMC article.
-
Increased Cellular Uptake of ApoE3- or c(RGD)-Modified Liposomes for Glioblastoma Therapy Depending on the Target Cells.Pharmaceutics. 2024 Aug 23;16(9):1112. doi: 10.3390/pharmaceutics16091112. Pharmaceutics. 2024. PMID: 39339149 Free PMC article.
-
SNX16 negatively regulates the migration and tumorigenesis of MCF-7 cells.Cell Regen. 2013 Apr 30;2(1):3. doi: 10.1186/2045-9769-2-3. eCollection 2013. Cell Regen. 2013. PMID: 25408875 Free PMC article.
-
LRP-1 and LRP-2 receptors function in the membrane neuron. Trafficking mechanisms and proteolytic processing in Alzheimer's disease.Front Physiol. 2012 Jul 16;3:269. doi: 10.3389/fphys.2012.00269. eCollection 2012. Front Physiol. 2012. PMID: 22934024 Free PMC article.
-
Clathrin and AP1B: key roles in basolateral trafficking through trans-endosomal routes.FEBS Lett. 2009 Dec 3;583(23):3784-95. doi: 10.1016/j.febslet.2009.10.050. Epub 2009 Oct 23. FEBS Lett. 2009. PMID: 19854182 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

