Experience leaves a lasting structural trace in cortical circuits
- PMID: 19005470
- PMCID: PMC6485433
- DOI: 10.1038/nature07487
Experience leaves a lasting structural trace in cortical circuits
Abstract
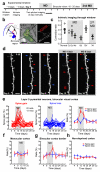
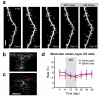
Sensory experiences exert a powerful influence on the function and future performance of neuronal circuits in the mammalian neocortex. Restructuring of synaptic connections is believed to be one mechanism by which cortical circuits store information about the sensory world. Excitatory synaptic structures, such as dendritic spines, are dynamic entities that remain sensitive to alteration of sensory input throughout life. It remains unclear, however, whether structural changes at the level of dendritic spines can outlast the original experience and thereby provide a morphological basis for long-term information storage. Here we follow spine dynamics on apical dendrites of pyramidal neurons in functionally defined regions of adult mouse visual cortex during plasticity of eye-specific responses induced by repeated closure of one eye (monocular deprivation). The first monocular deprivation episode doubled the rate of spine formation, thereby increasing spine density. This effect was specific to layer-5 cells located in binocular cortex, where most neurons increase their responsiveness to the non-deprived eye. Restoring binocular vision returned spine dynamics to baseline levels, but absolute spine density remained elevated and many monocular deprivation-induced spines persisted during this period of functional recovery. However, spine addition did not increase again when the same eye was closed for a second time. This absence of structural plasticity stands out against the robust changes of eye-specific responses that occur even faster after repeated deprivation. Thus, spines added during the first monocular deprivation experience may provide a structural basis for subsequent functional shifts. These results provide a strong link between functional plasticity and specific synaptic rearrangements, revealing a mechanism of how prior experiences could be stored in cortical circuits.
Figures


References
-
- Wiesel TN, Hubel DH. Single cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 1963;26:1003–1017. - PubMed
-
- Clark SA, Allard T, Jenkins WM, Merzenich MM. Receptive fields in the body-surface map in adult cortex defined by temporally correlated inputs. Nature. 1988;332:444–445. - PubMed
-
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Prior experience enhances plasticity in adult visual cortex. Nat. Neurosci. 2006;9:127–132. - PubMed
-
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu. Rev. Physiol. 1993;55:397–426. - PubMed
-
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu. Rev. Neurosci. 2001;24:1071–1089. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

