Illumination controls differentiation of dopamine neurons regulating behaviour
- PMID: 19005547
- PMCID: PMC2803045
- DOI: 10.1038/nature07569
Illumination controls differentiation of dopamine neurons regulating behaviour
Abstract
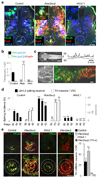
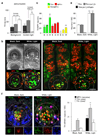
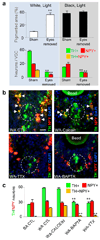
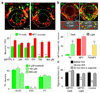
Specification of the appropriate neurotransmitter is a crucial step in neuronal differentiation because it enables signalling among populations of neurons. Experimental manipulations demonstrate that both autonomous and activity-dependent genetic programs contribute to this process during development, but whether natural environmental stimuli specify transmitter expression in a neuronal population is unknown. We investigated neurons of the ventral suprachiasmatic nucleus that regulate neuroendocrine pituitary function in response to light in teleosts, amphibia and primates. Here we show that altering light exposure, which changes the sensory input to the circuit controlling adaptation of skin pigmentation to background, changes the number of neurons expressing dopamine in larvae of the amphibian Xenopus laevis in a circuit-specific and activity-dependent manner. Neurons newly expressing dopamine then regulate changes in camouflage colouration in response to illumination. Thus, physiological activity alters the numbers of behaviourally relevant amine-transmitter-expressing neurons in the brain at postembryonic stages of development. The results may be pertinent to changes in cognitive states that are regulated by biogenic amines.
Figures


Comment in
-
Neuroscience: Light moulds plastic brains.Nature. 2008 Nov 13;456(7219):177-8. doi: 10.1038/456177a. Nature. 2008. PMID: 19005538 No abstract available.
References
-
- Brosenitsch TA, Katz DM. Expression of Phox2 transcription factors and induction of the dopaminergic phenotype in primary sensory neurons. Molecular and Cellular Neuroscience. 2002;20:447–457. - PubMed
-
- Borodinsky LN, et al. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

